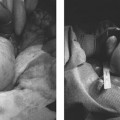
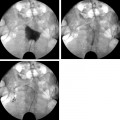
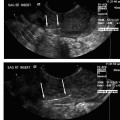
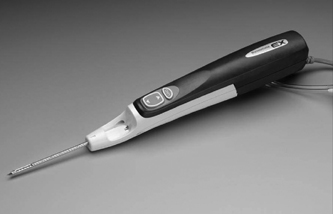
28 Forward Perspective: Interventional Radiology and the Breast Kenneth R. Tomkovich The field of interventional radiology is perhaps the most dynamic in all of medicine. Many of the procedures that are performed today were either nonexistent or heavily modified in the past 10 years. Uterine fibroid embolization, kyphoplasty, saphenous vein ablation, and many of the other procedures described in this text were not part of interventional radiology training programs in 1997. All of these as well as other procedures have been introduced, modified, or developed by interventional radiologists and incorporated into the practices and teaching programs during the past decade. The ability of interventional radiologists to adapt and apply new techniques and technology into their practices is probably the most important characteristic of this specialty. With the recent development of clinical practices and specialization in areas such as interventional oncology, new opportunities for growth and practice development have been presented to interventional radiologists. Using imaging guidance, interventional radiologists have become an integral member of the oncology service line. Procedures including percutaneous biopsies, radiofrequency and cryoablations, arterial chemoembolizations, yttrium-90 microsphere embolotherapy, and vascular access are all part of the services offered by interventional radiologists practicing oncologic interventions. Yet there is one glaring omission in the practice of most interventional radiologists: the practice of breast interventions. It is unclear the reason why the breast has been virtually omitted from the field of interventional radiology. As defined by the Society of Interventional Radiology, “Interventional Radiologists are board certified physicians who specialize in minimally invasive, targeted treatments performed using imaging for guidance. Their procedures have less risk, less pain and less recovery time compared to open surgery.”1 If this definition is accepted, then why omit care of the breast? In fact, the field of interventional radiology is well suited for breast care for many reasons. Interventional radiologists have training and expertise in performing biopsies safely and accurately in all areas of the body. Interventional radiologists have the most experience and expertise in percutaneous ablation procedures. Interventional radiologists may already care for some breast cancer patients through placement of vascular access for chemotherapy or through chemoembolization or radiofrequency ablation of metastatic disease. There are many procedures and applications of techniques unique to the breast, which can be learned and practiced by interventional radiologists. Once a lesion has been discovered, in any area of the body, a biopsy is required to direct treatment. Nowhere is this more important than in the breast. Patients with newly diagnosed breast lesions may have presented with a palpable growth or abnormality requiring imaging for further evaluation of the extent of disease or to look for disease in the contralateral breast.2 Most often, women present for annual screening mammography and have a newly diagnosed lesion requiring further assessment with imaging and then biopsy. The standard of care in 2007 is to perform image-guided breast biopsy if possible rather than performing open surgical biopsies.3 Patients will typically have a mammogram, which may or may not be combined with other imaging such as ultrasound or magnetic resonance imaging (MRI). Following the imaging workup, they will require a biopsy based on the interpretation of either a BI-RADS category 4 (suspicious) or 5 (highly suggestive of malignancy) abnormality. Some patients who have been given category 3 (probably benign) classifications are also presenting for biopsy in most cases because they are fearful of a growing lesion in their breast, may be planning pregnancy or breast augmentation procedures or have a breast cancer history or cancer in the opposite breast and need a more definitive diagnoses prior to therapy.3 It is this last point that is most important in understanding why percutaneous breast biopsy is the standard of care today. There are many options in treating breast cancer. The most obvious is surgical removal of the tumor. This can be accomplished through excisional biopsy, but more often than not this treatment is not definitive and patients would require a second open surgical procedure. The options presented to those with a percutaneous biopsy as the first diagnosis are many. Patients may have an additional, more detailed workup perhaps including a breast MRI prior to surgery. Patients may be given the options of neoadjuvant chemotherapy or hormone therapy prior to a surgical procedure in an attempt to shrink the size of the tumor and perhaps improve the surgical options for breast conservation. The tumor pathology can be accurately assessed including analysis of tumor markers such as estrogen and progesterone receptor sensitivity analysis. This can direct clinicians and patients with respect to the aggressiveness of the tumor and direct options for surgery and postsurgical care. Perhaps most importantly, percutaneous breast biopsy can be done safely and accurately in an outpatient setting avoiding the anxiety, extra time, and scarring, which are all associated with an open surgical procedure. Even with the improved accuracy of imaging and the use of digital mammography and MRI to help determine whether lesions of the breast are benign or malignant, nearly 80% of the biopsies recommended are still for benign lesions. It is important to be able to diagnose these benign breast lesions through percutaneous biopsy rather than open surgical procedures to avoid unnecessary surgery and scarring. In general, when planning for a percutaneous image-guided breast biopsy it is important to review the imaging to choose the best approach for the patient. Even if one does not read mammography, breast MRI, or ultrasound on a regular basis there is no reason that the interventional radiologist cannot participate and effectively perform these biopsies. Certainly, the majority of biopsy procedures in other organs such as the liver or lungs were diagnosed by other imagers and presented for review by the interventionalist. Likewise, breast lesions, detected by other imagers, can be evaluated for biopsy by clinicians who did not make the initial interpretation. There is no reason the breast should be treated differently. In addition, the fact remains that as of today, all interventional radiologists are still first diagnostic radiologists who had to pass the board examination, including the sections on mammography and breast imaging. Therefore, interventional radiologists are better suited than other specialists to participate in percutaneous breast interventions because of their background and training. As with any intervention, it is important to perform a brief history and physical examination prior to performing the procedure. Breast biopsies are no exception. It is very important to be aware of any medication or supplements such as aspirin, vitamin E, fish oils, or others that may predispose a patient to bleeding or hematoma, which is the most common complication from any percutaneous breast intervention.4 I recommend that a patient stop all such medication or supplements for 5 days prior to any intervention or biopsy. Options for biopsy guidance include stereotactic, ultrasound, and MRI, and perhaps in the near future digital tomosynthesis. Fine-needle aspiration (FNA) biopsy is often performed with ultrasound guidance. Fine-needle aspirations obtain a suspension of cells with a small gauge needle through a back and forth motion while aspirating on a syringe or trapping a column of cells in the needle through motion. These cells can then be processed and analyzed for cytologic features.5 This technique is not advisable except in situations where there is perhaps a mostly cystic lesion with or without a small solid or irregular component or in the case where there is a very large palpable lesion for confirmation of any already expected diagnosis. No tumor markers can be processed on FNA biopsies; often they are dependant on having a good histopathologist to process and interpret the findings. If performing FNA biopsies, it is most important to document concordance between the imaging findings and the pathology.5 Stereotactic breast biopsy is a mainstay of breast interventions. Lesions presenting as suspicious microcalcifications are most commonly approached with stereotactic guidance. Masses and areas of architectural distortion can also be biopsied with stereotactic technique. However, ultrasound guidance is the method of choice for these lesions if they can be identified sonographically. Lesions that are difficult to biopsy using stereotactic guidance are those which are very close to the chest wall, very superficial lesions, and those with very small or inconspicuous micro-calcifications.5 Stereotactic biopsy requires a dedicated table on which the patient lies in the prone position. The breast passes through a hole in the table and is positioned so as to best approach the lesion for biopsy. Through computer-generated coordinates based on position of the lesion relative to defined angles, the appropriate position and approach can be determined. Care should be taken to choose the shortest possible distance and to avoid any large or branching blood vessels, which may be near the biopsy site.5 Once the calculations are made as to the depth and position of the target area, the breast is prepped in sterile fashion. After administration of 5 to 10 cc of 1% lidocaine solution, a 3 mm incision is made through which an 8- or 11-gauge biopsy needle is advanced up to the lesion under stereotactic guidance as per the manufacturer’s instructions for depth. Confirmation imaging for needle placement is made and the needle is then deployed. Vacuum-assisted biopsy is then obtained from the site in a circumferential fashion. The number of cores obtained is dependent on what is being sampled and the gauge of the needle. I will typically obtain 12 cores using an 11-gauge needle and have most recently performed the majority of my stereotactic breast biopsies using an 8-gauge needle and only four cores. A specimen mammogram is suggested for all stereotactic biopsies particularly to document the removal of suspicious calcifications and to allow for separating the specimen into those with and without calcifications for pathologic analysis. After confirmation of the suspicious tissue in the biopsy specimen, a marker is placed at the biopsy site through the biopsy needle. I will typically close my incisions with ½ inch Steri-Strips (3M, St. Paul, MN) and apply a pressure dressing over the site. If there is bleeding at the biopsy site, it is important to maintain pressure to minimize the size of any breast hematoma and suture can be used to close the incision. Fig. 28.1 Image of the Mammotome EX Vacuum-assisted hand-held biopsy needle. (Courtesy Ethicon Endo-Surgery, Inc., Cincinnati, OH.) When a lesion is visible in the breast by ultrasound then percutaneous ultrasound-guided biopsy is the method of choice for obtaining a diagnostic sample of the tissue. The recent development of vacuum-assisted and stick freeze large-gauge hand-held biopsy devices have enabled clinicians to obtain larger samples under ultrasound guidance (Fig. 28.1). Although there may be a few circumstances in which FNA biopsy or spring-loaded core biopsy is performed in the breast, these are not the methods of choice for obtaining diagnostic samples and should not be utilized on a regular basis due to the availability, safety, and sample size of the vacuum-assisted or stick freeze large-gauge devices. Improper usage of FNA or small-gauge core needle biopsy can lead to a high percentage of false-negative biopsy results and unnecessary second biopsies due to discordance between imaging findings and pathology.6–9 Whenever possible, an 11-gauge or larger biopsy device should be used to perform ultrasound-guided core breast biopsy.10 The Cassi needle (Sanarus Medical, Pleasanton, CA) uses stick freeze technology. To perform this procedure, a 19-gauge guiding needle is advanced through the lesion under ultrasound guidance (Fig. 28.2A). An automated freezing procedure using a carbon dioxide cartridge freezes the 19-gauge needle to the lesion and an outer 10-gauge cutting needle advances over the 19-gauge needle in a circumferential manner to obtain the core biopsy sample (Fig. 28.2B). In my experience, usually no more than three passes are required to sample the lesion in question adequately. One disadvantage to this device is the requirement of withdrawing the needle after each pass to retrieve the sample, which requires reinsertion to obtain additional cores. The Cassi device has a sharp leading 19-gauge needle, which can be very helpful in gaining access to lesions in dense breast tissue or to lesions located near the chest wall or deep within the upper outer quadrant.10 Several vendors have developed vacuum-assisted core needle biopsy systems. These typically are made in 8-, 11-, and 14-gauge sizes. The benefit of these devices is that they require only one needle insertion to obtain multiple core samples from the lesion and due to the large cores obtained under direct ultrasound observation the number of discordant results are quite few. In fact, ultrasound-guided large-gauge vacuum-assisted core breast biopsy done with the proper technique in experienced hands is as accurate, or better than open breast biopsy performed after needle localization. In addition, a marker can be placed through most devices at the biopsy site.11 Vacuum-assisted biopsy has been shown to accurately diagnose atypical ductal hyperplasia, ductal carcinoma in situ, and microcalcifications.12–14 The breast is prepped and draped in sterile fashion. Local anesthetic is administered in the skin, along the biopsy tract, and distal to the biopsy site. A 3 mm incision is made through which the biopsy needle is advanced, under ultrasound guidance up to the lesion. The preferable approach is to have the needle under the mass so that the vacuum can draw the tissue into the aperture for biopsy, but this is not always possible (Fig. 28.3
Breast Biopsy
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree