Fractionated Radiation and the Dose-Rate Effect
▪ OPERATIONAL CLASSIFICATIONS OF RADIATION DAMAGE
Radiation damage to mammalian cells can operationally be divided into three categories: (1) lethal damage, which is irreversible and irreparable and, by definition, leads irrevocably to cell death; (2) potentially lethal damage (PLD), the component of radiation damage that can be modified by postirradiation environmental conditions; and (3) sublethal damage (SLD), which under normal circumstances, can be repaired in hours unless additional SLD is added (e.g., from a second dose of radiation) with which it can interact to form lethal damage (SLD repair, therefore, is manifested by the increase in survival observed if a dose of radiation is split into two fractions separated by a time interval).
Potentially Lethal Damage Repair
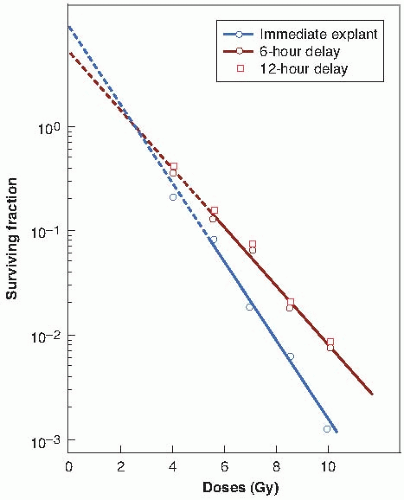
Varying environmental conditions after exposure to x-rays can influence the proportion of cells that survive a given dose because of the expression or repair of PLD. This damage is potentially lethal because under ordinary circumstances, it causes cell death, but if survival is increased as a result of the manipulation of the postirradiation environment, PLD is considered to have been repaired. PLD is repaired if cells are incubated in a balanced salt solution instead of a full growth medium for several hours after irradiation. This is a drastic treatment, however, and does not mimic a physiologic condition that is ever likely to occur. Little and his colleagues chose to study PLD repair in density-inhibited stationary-phase cell cultures, which are considered a better in vitro model for tumor cells in vivo (Fig. 5.1). Cell survival was enhanced considerably if the cells were allowed to remain in the density-inhibited state for 6 or 12 hours after irradiation before being subcultured and assayed for colony-forming ability.
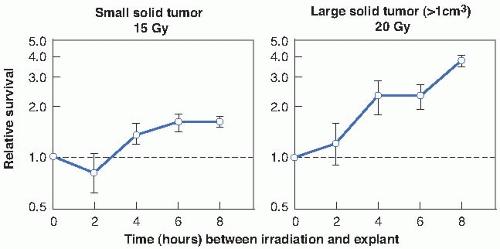
The relevance of PLD to radiotherapy became much more obvious when it was shown that repair, comparable in magnitude and kinetics to that found in vitro, also occurred in vivo in experimental tumors. In this case, repair took the form of significantly enhanced cell survival if several hours were allowed to elapse between irradiation of the tumor in situ and removal of the cells from the host to assess their reproductive integrity (Fig. 5.2).
To summarize the available experimental data, there is a general agreement that PLD is repaired, and the fraction of cells surviving a given dose of x-rays is enhanced if postirradiation conditions are suboptimal for growth, so that cells do not have to attempt the complex process of mitosis while their chromosomes are damaged. If mitosis is delayed by
suboptimal growth conditions, DNA damage can be repaired.
suboptimal growth conditions, DNA damage can be repaired.
The importance of PLD repair to clinical radiotherapy is a matter of debate. That it occurs in transplantable animal tumors has been documented beyond question, and there is no reason to suppose that it does not occur in human tumors. It has been suggested that the radioresistance of certain types of human tumors is linked to their ability to repair PLD; that is, radiosensitive tumors repair PLD inefficiently, but radioresistant tumors have efficient mechanisms to
repair PLD. This is an attractive hypothesis, but it has never been proven.
repair PLD. This is an attractive hypothesis, but it has never been proven.
Sublethal Damage Repair
SLD repair is the operational term for the increase in cell survival that is observed if a given radiation dose is split into two fractions separated by a time interval.
Figure 5.3 shows data obtained in a splitdose experiment with cultured Chinese hamster cells. A single dose of 15.58 Gy of absorbed radiation leads to a surviving fraction of 0.005. If the dose is divided into two approximately equal fractions separated by 30 minutes, the surviving fraction is already appreciably higher than for a single dose. As the time interval is extended, the surviving fraction increases until a plateau is reached at about 2 hours, corresponding to a surviving fraction of 0.02. This represents about four times as many surviving cells as for the dose given in a single exposure. A further increase in the time interval between the dose fractions is not accompanied by any significant additional increment in survival. The increase in survival in a split-dose experiment results from the repair of sublethal radiation damage.
The data shown in Figure 5.3 were obtained with cultured mammalian cells maintained at room temperature (24° C) between the dose fractions to prevent the cells from moving through the cell cycle during this interval. This rather special experiment is described first because it illustrates repair of sublethal radiation damage uncomplicated by the movement of cells through the cell cycle.
Figure 5.4 shows the results of a parallel experiment in which cells were exposed to split doses and maintained at their normal growing temperature of 37° C. The pattern of repair seen in this case differs from that observed for cells kept at room temperature. In the first few hours, prompt repair of SLD is again evident, but at longer intervals between the two split doses, the surviving fraction of cells decreases, reaching a minimum with about a 5-hour separation.
An understanding of this phenomenon is based on the age-response function described in Chapter 4. If an asynchronous population of cells is exposed to a large dose of radiation, more cells are killed in the sensitive than in the resistant phases of the cell cycle. The surviving population of cells, therefore, tends to be partly synchronized.
In Chinese hamster cells, most of the survivors from a first dose of radiation are located in the S phase of the cell cycle. If about 6 hours are allowed to elapse before a second dose of radiation is given, this cohort of cells progresses around the cell cycle and is in G2/M, a sensitive period of the cell cycle at the time of the second dose. If the increase in radiosensitivity in moving from late S to the G2/M period exceeds the effect of repair of SLD, the surviving fraction falls.
The pattern of repair shown in Figure 5.4 is therefore a combination of three processes occurring simultaneously. First, there is the prompt repair of sublethal radiation damage. Second, there is progression of cells through the cell cycle during the interval between the split doses, which has been termed reassortment. Third, there is an increase of surviving fraction resulting from cell division, or repopulation, if the interval between the split doses is from 10 to 12 hours, because this exceeds the length of the cell cycle of these rapidly growing cells.
This simple experiment, performed in vitro, illustrates three of the “four Rs” of radiobiology: repair, reassortment, and repopulation. The fourth “R,” reoxygenation, is discussed in Chapter 6. It should be emphasized that the dramatic dip in the split-dose curve at 6 hours caused by reassortment, and the increase in survival by 12 hours because of repopulation are seen only for rapidly growing cells. Hamster cells in culture have a cycle time of only 9 or 10 hours. The time sequence of these events would be longer in more slowly proliferating normal tissues in vivo.
Repair of sublethal radiation damage has been demonstrated in just about every biologic test system for which a quantitative end point is available. Figure 5.5 illustrates the pattern for repair of sublethal radiation damage in two in vivo systems in mice, P388 lymphocytic leukemia and skin cells. In neither case, there is a dramatic dip in the curve at 6 hours resulting from movement of cells through the cycle, because the cell cycle is long. In resting skin, for example, the cell cycle of stem cells may be as long as 10 days rather than 9 hours of the rapidly growing cells in Figure 5.4. The mouse tumor data show more repair in small 1-day tumors than in large hypoxic 6-day tumors; this important point illustrates that repair is an active process requiring oxygen and nutrients.
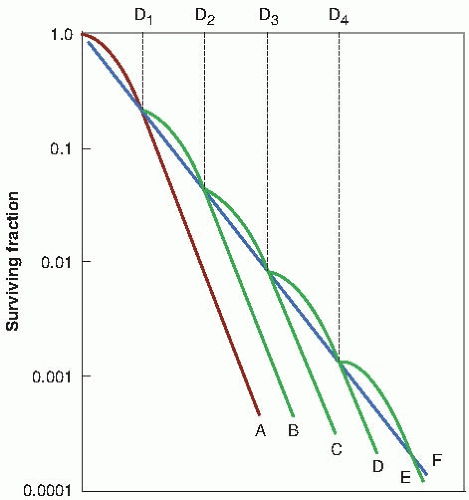
The various factors involved in the repair of SLD are summarized in Figure 5.6. Figure 5.6A shows that if a dose is split into two fractions separated by a time interval, more cells survive than for the same total dose given in a single fraction, because the shoulder of the curve must be repeated with each fraction. In general, there is a good correlation between the extent of repair of SLD and the size of the shoulder of the survival curve. This is not surprising because both are manifestations of the same basic phenomenon: the accumulation and repair of SLD. Some mammalian cells are characterized by a survival curve with a broad shoulder, and split-dose experiments then indicate a substantial amount of SLD repair. Other types of cells show a survival curve with a minimal shoulder, and this is reflected in more limited repair of SLD. In the terminology of the linear-quadratic (α/β) description of the survival curve, it is the quadratic component (β) that causes the curve to bend and that results in the sparing effect of a split dose. A large shoulder corresponds to a small α/β ratio.
 FIGURE 5.6 Summary of the repair of sublethal damage as evidenced by a splitdose experiment. A: If the dose is delivered in two fractions separated by a time interval, there is an increase in cell survival because the shoulder of the curve must be expressed each time. B: The fraction of cells surviving a split dose increases as the time interval between the two dose fractions increases. As the time interval increases from 0 to 2 hours, the increase in survival results from the repair of sublethal damage. In cells with a long cell cycle or that are out of cycle, there is no further increase in cell survival by separating the dose by more than 2 or 3 hours. In a rapidly dividing cell population, there is a dip in cell survival caused by reassortment. However, as shown in Figure 5.7, if the time interval between the split doses exceeds the cell cycle, there is an increase in cell survival owing to proliferation or repopulation between the doses. |
The time course of the increase in cell survival that results from the repair of SLD is charted in Figure 5.6B. As the time interval between the two dose fractions is increased, there is a rapid increase in the fraction of cells surviving owing to the prompt repair of SLD. This repair is complete by 1 or 2 hours for cells in culture but may take longer for late-responding tissues in vivo ( Chapter 23). As the time interval between the two dose fractions is increased, there is a dip in the curve owing to the movement of surviving cells through the cell cycle, as explained in Figure 5.4. This occurs only in a population of fast- cycling cells. In cells that are noncycling, there can be no dip. If the time interval between the two dose
fractions exceeds the cell cycle, there is an increase in the number of cells surviving because of cell proliferation; that is, cells can double in number between the dose fractions.
fractions exceeds the cell cycle, there is an increase in the number of cells surviving because of cell proliferation; that is, cells can double in number between the dose fractions.
▪ MECHANISM OF SUBLETHAL DAMAGE REPAIR
In Chapter 3, evidence was summarized of the correlation between cell killing and the production of asymmetric chromosomal aberrations, such as dicentrics and rings. This, in turn, is a consequence of an interaction between two (or more) double-strand breaks in the DNA. Based on this interpretation, the repair of SLD is simply the repair of double-strand breaks. If a dose is split into two parts separated by a time interval, some of the double-strand breaks produced by the first dose are rejoined and repaired before the second dose. The breaks in two chromosomes that must interact to form a lethal lesion such as a dicentric may be formed by (1) a single track breaking both chromosomes (i.e., singletrack damage), or (2) separate tracks breaking the two chromosomes (i.e., multiple-track damage).
The component of cell killing that results from single-track damage is the same whether the dose is given in a single exposure or fractionated. The same is not true of multiple-track damage. If the dose is given in a single exposure (i.e., two fractions with t = 0 between them), all breaks produced by separate electrons can interact to form dicentrics. But if the two dose fractions (D/2) are separated by, for example, 3 hours, then breaks produced by the first dose may be repaired before the second dose is given. Consequently, there are fewer interactions between broken chromosomes to form dicentrics, and more cells survive. Based on this simple interpretation, the repair of SLD reflects the repair and rejoining of double-strand breaks before they can interact to form lethal lesions. This may not be the whole story, but it is a useful picture to keep in mind.
▪ REPAIR AND RADIATION QUALITY
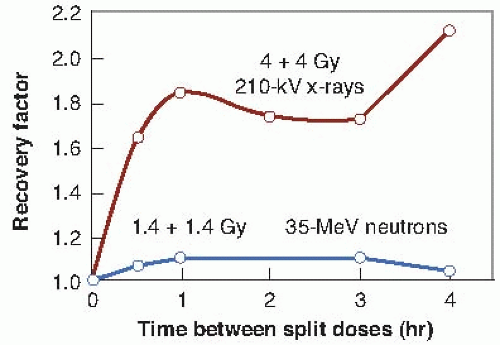
For a given biologic test system, the shoulder on the acute survival curve and, therefore, the amount of SLD repair indicated by a split-dose experiment vary with the type of radiation used. The effect of dose fractionation with x-rays and neutrons is compared in Figure 5.7. For x-rays, dividing the total dose into two equal fractions, separated from 1 to 4 hours, results in a marked increase in cell survival because of the prompt repair of SLD. By contrast, dividing the dose into two fractions has little effect on cell survival if neutrons are used, indicating little repair of SLD.
▪ THE DOSE-RATE EFFECT
For x- or γ-rays, dose rate is one of the principal factors that determine the biologic consequences of a given absorbed dose. As the dose rate is lowered and the exposure time extended, the biologic effect of a given dose generally is reduced.
The classic dose-rate effect, which is very important in radiotherapy, results from the repair of SLD that occurs during a long radiation exposure. To illustrate this principle, Figure 5.8 shows an idealized experiment in which each dose (D2, D3, D4, and so on) is delivered in several equal fractions of size D, with a time interval between fractions that is sufficient for repair of SLD. The shoulder of the survival curve is repeated with each fraction. The broken line, F, shows the overall survival curve that would be
observed if only single points were determined, corresponding to equal dose increments. This survival curve has no shoulder. Because continuous low-dose-rate (LDR) irradiation may be considered to be an infinite number of infinitely small fractions, the survival curve under these conditions also would be expected to have no shoulder and to be shallower than for single acute exposures.
observed if only single points were determined, corresponding to equal dose increments. This survival curve has no shoulder. Because continuous low-dose-rate (LDR) irradiation may be considered to be an infinite number of infinitely small fractions, the survival curve under these conditions also would be expected to have no shoulder and to be shallower than for single acute exposures.
▪ EXAMPLES OF THE DOSE-RATE EFFECT IN VITRO AND IN VIVO
Survival curves for HeLa cells cultured in vitro over a wide range of dose rates, from 7.3 Gy/min to 0.535 cGy/min, are summarized in Figure 5.9. As the dose rate is reduced, the survival curve becomes shallower and the shoulder tends to disappear (i.e., the survival curve becomes an exponential function of dose). The dose-rate effect caused by repair of SLD is most dramatic between 0.01 and 1 Gy/min. Above and below this dose-rate range, the survival curve changes little, if at all, with dose rate.
The magnitude of the dose-rate effect from the repair of SLD varies enormously among different types of cells. HeLa cells are characterized by a survival curve for acute exposures that has a small initial shoulder, which goes hand in hand with a modest dose-rate effect. This is to be expected, because both are expressions of the cell’s capacity to accumulate and repair sublethal radiation damage. By contrast, Chinese hamster cells have a broad shoulder to their acute x-ray survival curve and show a correspondingly large dose-rate effect. This is evident in Figure 5.10;
there is a clear-cut difference in biologic effect, at least at high doses, between dose rates of 1.07, 0.30, and 0.16 Gy/min. The differences between HeLa and hamster cells in the size of the shoulder to the acute survival curve and the magnitude of the dose-rate effect reflect differences in the importance of apoptosis. In the case of HeLa cells, apoptosis is an important form of cell death following radiation, whereas for hamster cells, apoptotic death is rarely seen.
there is a clear-cut difference in biologic effect, at least at high doses, between dose rates of 1.07, 0.30, and 0.16 Gy/min. The differences between HeLa and hamster cells in the size of the shoulder to the acute survival curve and the magnitude of the dose-rate effect reflect differences in the importance of apoptosis. In the case of HeLa cells, apoptosis is an important form of cell death following radiation, whereas for hamster cells, apoptotic death is rarely seen.
 FIGURE 5.9 Survival curves for HeLa cells cultured in vitro and exposed to γ-rays at high and low dose rates. |
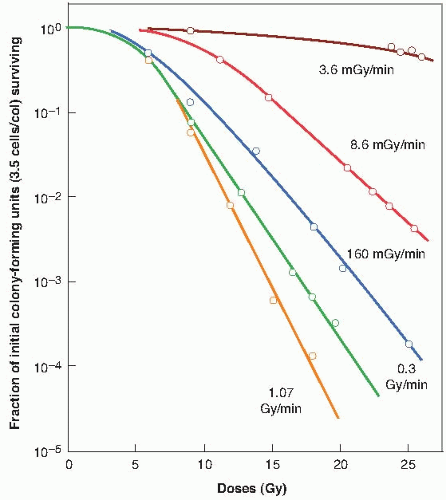 FIGURE 5.10 Dose-response curves for Chinese hamster cells (CHL-F line) grown in vitro
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|