Patient characteristics
Age (years)
68 (47–82)
Male/female
26/16
Tumor location
Upper/middle/lower
5/23/14
Less/ant/post/gre
24/5/3/10
Pathological depth of invasion
T1a(M)/T1b(SM)/T2(MP)/T3(SS)
16/17/8/1
Tumor size after gastrectomy (mm)
27 (10–65)
Surgical approach
Open/laparoscopic
15/27
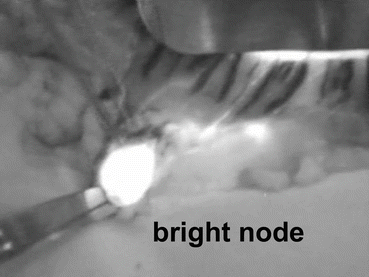
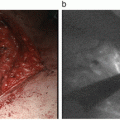
Fig. 13.1
Bright node detected by ICG fluorescence imaging with the Photodynamic Eye. An intraoperative image obtained after distal partial gastrectomy with standard lymph node dissection up to D2 is shown. The tumor is seen at the greater curvature of the middle third of the stomach. Fluorescence is noted in the lymphatics and lymph nodes. In this case, the lymphatic basin is in the right gastroepiploic artery area, and bright nodes are No. 4d
Table 13.2
Bright node biopsy results
Bright node biopsy | Status of all dissected nodes | |
|---|---|---|
Metastasis | No metastasis | |
Metastasis | 7 | 0 |
No metastasis | 0 | 35 |
13.6 Unsolvable Issues of ICG Fluorescence Mapping
Our results suggested that ICG fluorescence imaging for SN biopsy might be feasible in both open and laparoscopic surgery for early gastric cancers. On the other hand, some disadvantages of ICG fluorescence imaging were also obvious in our analysis, the subjectivity of SN evaluation and potential secondary node contamination because of the high sensitivity of the PDE system.
In the preliminary phase of our study, we attempted intraoperative mapping. However, intraoperative mapping was difficult because of pollution of the surgical field by ICG leakage from the edge of the dissected lymphatic basin (data not shown). A reduction in ICG dose is essential when performing SN biopsy for gastric cancer, as this region has a rich lymphatic flow. We thought that the PDE was sensitive enough to detect minute ICG concentrations. We next changed the timing of injection from intraoperative to preoperative (the day before surgery). A marked reduction in the pollution of the surgical field was achieved with this modification. The adequate concentration of ICG was determined in the initial eight cases, which were all node negative. Table 13.3 shows the relationship between the number of bright nodes and ICG concentration. At an ICG concentration of 5 μg/mL (×1000), bright lymphatics and bright nodes were detected despite the weak fluorescence. Therefore, the adequate concentration of ICG was determined to be 50 μg/mL (×100). Next, we determined the adequate injection volume. Fifteen and 19 sequential patients were injected with 0.5 mL × 4 points and 0.2 mL × 4 points of 50 μg/mL ICG, respectively. The visualization of bright nodes and bright lymphatics was similar between the two groups. The median number of bright nodes was not significantly different between the two groups. Therefore, the adequate injection volume of ICG solution was determined to be 0.5 mL × 4 points because of its technical reliability.
Table 13.3
Relationship between the number of bright nodes and ICG concentration
Concentration | Volume | No. of cases | No. of bright nodes (median) |
|---|---|---|---|
×2 (2.5 mg/mL) | 0.5 mL × 4 | 1 | 17 |
×40 (125 μg/mL) | 0.5 mL × 4 | 2 | 8 (8, 8) |
×100 (50 μg/mL) | 0.5 mL × 4 | 4 | 5 (4–7) |
×1000 (5 μg/mL) | 0.5 mL × 4 | 1 | 3 |
×100 (50 μg/mL) | 0.5 mL × 4 | 15 | 6 (3–11) |
×100 (50 μg/mL) | 0.2 mL × 4 | 19 | 6 (2–7) |
The numbers of bright nodes depended on the concentration of the ICG solution injected. Therefore, the bright nodes detected by the PDE seemed to contain a few secondary nodes. Secondary node contamination is a significant drawback of ICG fluorescence imaging resulting from its high sensitivity, because true SNs are difficult to distinguish from secondary nodes. In the radioisotope method, the threshold for distinguishing true SNs from secondary nodes is determined by measuring the radioactive count of hot nodes. This is because the SNs receive much greater lymphatic flow and take up more radioactive colloid than secondary nodes. We hypothesize that ICG-lipoprotein complexes might behave in the same manner as radioactive colloids and, therefore, the threshold level for true SN detection could be determined by measuring ICG fluorescence intensity. Further investigation is needed to validate our hypothesis and to overcome this limitation of ICG fluorescence imaging.
13.7 The Future of Laparoscopic Gastric Surgery
At present, laparoscopic gastrectomy merely replicates standard gastrectomy. It is difficult to perform function-preserving curative gastrectomy using the laparoscopic approach. However, if ICG fluorescence mapping for early gastric cancer becomes feasible, laparoscopic function-preserving curative gastrectomy would be a good alternative to the commonly used D1+ gastrectomy for patients with node-negative gastric cancer. Schematic diagrams of function-preserving gastrectomy with lymphatic basin dissection are shown in Fig. 13.2.
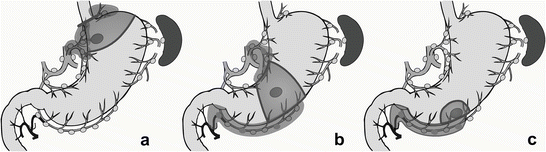

Fig. 13.2
Schematic diagrams of function-preserving gastrectomy with lymphatic basin dissection. (a) Limited proximal gastrectomy, (b) segmental gastrectomy, (c) local resection
Our results were obtained using the PDE, so a small incision at the upper abdomen was needed to assess the direction of the lymphatic basins. However, several ICG fluorescence imaging systems have been developed and are available, including the IMAGE 1 HD system by Karl Storz, the IRI system by Olympus, the ICG fluorescent laparoscope by Shinko-Optical, and the PINPOINT system by Novadaq. The use of these systems would allow avoidance of the upper abdomen incision and promote widespread proliferation of ICG fluorescence imaging and function-preserving curative gastrectomy. Nevertheless, the proper concentration of ICG should be reexamined if these detection devices are to be used.
A limitation of ICG fluorescence imaging is the subjectivity of SN evaluation and potential secondary node contamination. Nevertheless, the elimination of secondary node contamination will be possible by using new agents. New fluorescence agents with both fluorescence and colloid particle characteristics have been developed [38–42]. These agents detect only fluorescent SNs and not secondary nodes and could potentially be useful in laparoscopic SN biopsy in cases of gastric cancer. Furthermore, they may be used alone as a standard tracer instead of in combination with other SN mapping tracers.
References
1.
Gotoda T, Yamamoto H, Soetikno RM et al (1999) Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol 41:929–942CrossRef
2.
Japanese Gastric Cancer Association (2011) Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 14:113–123CrossRef
3.
Eagon JC, Miedema BW, Kelly KA (1992) Postgastrectomy syndromes. Surg Clin N Am 72:445–465PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree