Basic Principles
CT complements endoscopy and barium examination of the gastrointestinal (GI) tract by demonstration of intramural and extraintestinal components of GI disease, including disease in the mesentery, peritoneal cavity, lymph nodes, and liver. CT is used to diagnose the presence of GI disease, evaluate its nature and extent, and demonstrate complications such as abscess, phlegmon, fistula, and perforation. CT is excellent for determining the extent of GI disease but is less often specific for its nature.
The GI tract is shown on every CT of the abdomen. The intestinal lumen should be distended and opacified for routine abdominal CT by administration of 700 to 800 mL of 2% to 3% iodinated, or barium, contrast agent at least 1 hour before routine scanning. Water is an excellent contrast agent for the lumen of the stomach and upper intestinal tract and can be used whenever disease in the upper abdomen is suspected. Intravenous contrast medium is used to assess enhancement of the GI mucosa and of lesions, to demonstrate blood vessels, and to evaluate the solid organs of the abdomen. Thin-section scans improve lesion definition. The short scan times of multidetector CT (MDCT) improve image quality by limiting motion artifact. Collapsed bowel loops without intraluminal contrast enhancement may mimic adenopathy and mass lesions. However, when scans are obtained during the arterial phase of bolus contrast medium enhancement, identification of enhanced bowel wall confirms the nature of nondistended bowel.
A CT hallmark of intestinal disease is thickening of the bowel wall. When fully distended, the bowel wall is 1 to 2 mm thick. When collapsed, the bowel wall should not exceed 3 to 4 mm, except in the stomach near the esophageal junction, where the normal stomach wall may be 2 cm thick when collapsed. The CT appearance of wall thickening is helpful in differentiating benign wall thickening from malignant wall thickening ( Table 17.1 ). Benign wall thickening usually does not exceed 1 cm, is homogeneous in attenuation, and is circumferential, symmetric, and segmental in distribution. The double halo and target appearance of the intestine in cross section is caused by inflammation, edema, and hyperemia and is best demonstrated on contrast-enhanced scans. Neoplastic wall thickening is thicker (1–2 cm), asymmetric, nodular, lobulated, or spiculated in contour and tends to narrow the intestinal lumen. Benign wall thickening is caused by inflammatory bowel disease, intestinal ischemia, and intramural hemorrhage. Neoplastic wall thickening is produced by adenocarcinoma, lymphoma, and GI stromal tumors (GISTs).
| Benign | Malignant |
|---|---|
| Homogeneous attenuation | Heterogeneous attenuation |
| Symmetric | Asymmetric |
| Circumferential | Eccentric |
| Thickening <1 cm | Thickening >1–2 cm |
| Segmental or diffuse involvement | Focal mass |
| “Double-halo sign” | Abrupt transition |
| Dark inner ring | Lobulated contour |
| Bright outer ring | Spiculated contour |
| “Target sign” | Narrowed bowel lumen |
| Bright inner ring | Enlarged lymph nodes |
| Dark middle ring | Liver metastases |
| Bright outer ring |
CT Enterography
CT enterography using MDCT is a first-line modality for examination of small bowel disease. CT enterography differs from routine CT of the abdomen and pelvis by use of large volumes of low-attenuation intraluminal contrast agent to optimally distend the bowel lumen, matched with thin-slice collimation and routine high-detail coronal and sagittal reformations. Low-attenuation intraluminal contrast agent paired with intravenous contrast agent administration optimally displays both the lumen and the wall of the small bowel ( Fig. 17.1 ). The advantages of CT enterography over traditional barium-based small bowel follow-through examination include demonstration of the entire thickness of the bowel wall and disease in the mesentery, as well as display of bowel loops without superimposition. The indications for CT enterography include Crohn disease and other suspected inflammatory bowel diseases, intermittent small-bowel obstruction, obscure GI bleeding, and suspected tumors of small bowel. A typical protocol for CT enterography includes:
- •
A minimum 4-hour fast.
- •
Administration of low-attenuation (20 Hounsfield units [HU]) oral contrast agent, typically VoLumen, a low-concentration barium sulfate suspension, 0.1% w/v (Bracco E-Z-EM). A total volume 1400 mL is given in divided doses: 450 mL 60 minutes before scanning, 450 mL 40 minutes before scanning, 250 mL 20 minutes before scanning, and 250 mL 10 minutes before scanning. Glucagon (0.5 mg) may be given intramuscularly to inhibit bowel motion.
- •
Administration of 125 mL of 60% iodine intravenous contrast agent at 3 to 4 mL per second provides enhancement of the bowel wall as well as of any lesions.
- •
MDCT scanning is performed from the dome of diaphragm through the ischial tuberosities with 0.625-mm collimation with reconstructions at 2.5-mm slice thickness.
- •
For inflammatory bowel disease or other diffuse bowel disease, images are obtained after contrast agent administration only in the portal venous phase with an 80-second delay.
- •
For occult GI bleeding or suspected GI malignancy, scans are obtained in the arterial phase (30-second scan delay), portal venous phase (80-second scan delay), and delayed phase (3-minute scan delay).
- •
Images are reconstructed in axial, coronal, and sagittal planes.

CT Enteroclysis
CT enteroclysis is performed on patients with small-bowel obstruction to find the level and cause of obstruction ( Fig. 17.2 ). For CT enteroclysis, contrast medium for small-bowel distention is injected into the small bowel through a nasojejunal tube rather than given orally as for CT enterography. A nasojejunal tube is positioned at the duodenojejunal junction under fluoroscopic guidance. A total of 1200 to 1600 mL of low-attenuation oral contrast medium is injected into the small bowel at 60 to 120 mL per minute. Glucagon, or other antispasmodic medication, is administered. Intravenous contrast agent is used, and MDCT scanning is performed with the same parameters as for CT enterography.

CT Colonography
CT colonography is used to investigate the colon for colorectal polyps and cancers. CT colonography is now the radiologic examination of choice for the diagnosis of colorectal malignancy. Numerous studies have shown that CT colonography is nearly equal to traditional optical colonoscopy for detecting cancer and precancerous lesions. Many patients prefer CT colonography over colonoscopy because of perceived safety and convenience. The obvious disadvantage of CT colonography is for that lesions that require biopsy, the patient will have to undergo subsequent colonoscopy.
- •
Standard bowel preparation, similar to that used for colonoscopy, is begun the day before the examination.
- •
Barium sulfate and iodinated contrast medium solutions are given orally to tag remaining stool and colonic fluid.
- •
For the examination a catheter is placed in the rectum and the colon is insufflated with an automated carbon dioxide system that provides pressure and volume regulation.
- •
MDCT scanning is performed with 1.25-mm collimation and a low-radiation dose protocol with the patient in both the prone and the supine position.
- •
Images are reviewed on a workstation that provides three-dimensional volume rendering with endoluminal display and “fly-through” capability ( Fig. 17.3 ).

FIG. 17.3
CT colonography.
Image from a three-dimensional work station used for interpretation of CT colonography examinations showing the location of a polyp (yellow arrowhead) in the splenic flexure of the colon 121.6 cm from the anal verge. Accurate landmarks ( yellow arrows and blue markers ) allow rapid localization of the polyp for removal by colonoscopy.
Esophagus
Anatomy
The esophagus is a muscular tube that extends from the cricopharyngeus muscle at the level of the cricoid cartilage to the stomach. The major portion of its length is within the middle mediastinum. The cervical portion extends from the level of the C6 vertebral body to the thoracic inlet. A short abdominal segment extends below the diaphragm to the gastroesophageal junction. The esophagus is lined by squamous epithelium to the gastric junction, where the mucosa abruptly changes to columnar epithelium. The lack of serosal covering allows early invasion by esophageal tumors into periesophageal tissues. The musculature of the esophageal wall is striated in the upper third, striated and smooth muscle in the middle third, and solely smooth muscle in the distal third.
On CT the esophagus appears as an oval of soft-tissue density often containing air or contrast material within its lumen ( Fig. 17.4 ). When distended, the wall of the esophagus should not exceed 3 mm in thickness. In the neck and upper thorax the esophagus courses between the trachea and the spine. In the lower thorax the esophagus courses to the right of the descending aorta between the left atrium and the spine. The esophagus enters the abdomen through the esophageal hiatus and courses to the left to join the stomach. The edges of the diaphragmatic crura forming the esophageal hiatus are seen as often prominent, teardrop-shaped structures partially surrounding the esophagus. Air columns in the esophagus longer than 10 to 15 cm may be evidence of stricture or impaired peristalsis. Air-fluid levels in the esophagus are always abnormal.

Esophageal Carcinoma
Because of the lack of serosal covering, carcinoma spreads beyond the esophagus early in its course, resulting in a poor prognosis. Ninety percent of tumors are squamous cell carcinoma, with the remaining 10% being adenocarcinoma arising in Barrett esophagus in the distal esophagus. The CT findings in esophageal carcinoma may be duplicated by benign disease. Diagnosis depends on biopsy. CT is performed to assess the extent of disease and to identify those patients whose disease cannot be surgically resected. The CT findings in esophageal carcinoma include the following:
- •
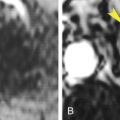
Irregular thickening of the wall of the esophagus of more than 3 mm ( Fig. 17.5 ).

FIG. 17.5
Adenocarcinoma: esophagus.
(A) Coronal reconstruction CT image showing distension of the normal upper esophagus (red arrow) with air caused by abrupt narrowing (yellow arrowhead ) of the lumen of the mid esophagus. The stomach (S) is evident below the diaphragm. (B) Axial CT image at the level of the stricture showing circumferential thickening of the wall of the esophagus (arrowhead) , with narrowing of its lumen marked by a small pocket of air. Biopsy revealed adenocarcinoma in a long segment of Barrett esophagus. Ao , Thoracic aorta; Ht , heart.
- •
Intraluminal polypoid mass.
- •
Eccentric narrowing of the lumen.
- •
Dilatation of the esophagus above the area of narrowing with air or fluid.
- •
Invasion of periesophageal tissues: fat, aorta, trachea.
- •
Tumor invasion of the trachea or bronchi is suggested by tumor that displaces or indents the posterior airway wall (90% accurate).
- •
Tumor invasion of the aorta is suggested by an arc of contact between the tumor and aorta of greater than 90 degrees. An arc less than 45 degrees indicates no invasion, and an arc between 45 and 90 degrees is indeterminate. These findings are about 80% accurate.
- •
Metastases to lymph nodes, liver, and other organs.
- •
Esophageal carcinoma spreads to paraesophageal, other mediastinal, gastrohepatic ligament, and left gastric nodal chains ( Fig. 17.6 ). Microscopic disease in normal-sized nodes, and lymph node enlargement as a result of benign conditions, limits the CT accuracy of nodal involvement by esophageal carcinoma to 39% to 85%.

FIG. 17.6
Carcinoma of the gastroesophageal junction.
Carcinoma arising near the gastroesophageal junction has spread to the liver (curved yellow arrow) and to lymph nodes (yellow arrowheads) surrounding the celiac axis (straight red arrow) .
- •
Tumor recurrence after esophagectomy is well demonstrated by CT. Tumors may recur anywhere within the mediastinum, in distant lymph nodes in the neck or abdomen, and in the liver, lung, pleural space, adrenal glands, or peritoneal cavity.
Esophageal Mesenchymal Tumors
Leiomyoma is the most common benign tumor of the esophagus. Most smooth muscle tumors of the esophagus are true leiomyomas. GISTs are rare tumors of the esophagus, occurring more commonly in the stomach and small intestine. Immunohistologic studies differentiate the two tumors. Most esophageal mesenchymal tumors are asymptomatic until they become very large and cause dysphagia. Endoscopy demonstrates a submucosal mass, usually easily differentiated from carcinoma.
- •
On CT, leiomyoma appears as a smooth, well-defined 2- to 8-cm mass of uniform soft-tissue attenuation. The esophageal wall is eccentrically thickened, and the lumen is deformed. A large, well-defined mass is much more likely to be a leiomyoma than a carcinoma. Leiomyomas occurs multiply in about 4% of cases.
- •
Leiomyomas, and rarely GISTs, are the only tumors of the esophagus that may have coarse calcifications ( Fig. 17.7 ).

FIG. 17.7
Leiomyoma of the esophagus.
Axial CT in a patient with dysphagia reveals a large tumor (between red arrowheads ) with coarse calcifications eccentrically narrowing and markedly displacing the lumen (yellow arrow) of the esophagus. Surgical removal confirmed a benign leiomyoma. Ao , Thoracic aorta; RA , right atrium of the heart.
- •
Compared with leiomyomas, GISTs appear more heterogeneous, tend to be larger, and enhance more avidly on CT. GISTs are markedly avid for 18 F-fludeoxyglucose (FDG) on positron emission tomography. Esophageal leiomyomas are infrequently FDG avid.
- •
Both leiomyomas and GISTs have malignant potential. Leiomyosarcomas tend to grow intraluminally, are usually large (>5 cm), have heterogeneous attenuation, and may ulcerate.
Esophageal Varices
Esophageal varices are most often caused by portal hypertension but may occur with superior vena cava obstruction. The major complication is hemorrhage.
- •
Varices are clearly recognized on postcontrast CT as well-defined enhancing nodular and tubular densities adjacent to the esophagus and within its wall ( Fig. 17.8 ).

FIG. 17.8
Esophageal varices.
Numerous large enhancing varices (red arrowheads) resulting from cirrhosis and portal hypertension surround and indent the distal esophagus (yellow arrow) . (A) Axial postcontrast CT. (B) Coronal postcontrast CT in the same patient. Ao , Descending thoracic aorta.
- •
Varices cause scalloped thickening of the esophageal wall that may be indistinguishable from tumor or inflammation without the use of contrast enhancement.
- •
Signs of cirrhosis, portal hypertension, and other portosystemic collateral vessels are usually present.
Esophagitis
The causes of esophagitis include gastroesophageal reflux, corrosive ingestion, long-term intubation, radiation, and infection. Infectious esophagitis is most commonly seen in immunosuppressed patients. Causative organisms include Candida , herpes simplex virus, cytomegalovirus, and Mycobacterium tuberculosis.
- •
The major CT finding of severe esophagitis is a relatively long segment of circumferential symmetric wall thickening (>5 mm) ( Fig. 17.9 ), often with mucosal enhancement.

FIG. 17.9
Esophagitis.
Reflux esophagitis causes circumferential thickening of the wall of the distal esophagus (arrowheads) . (A) Axial noncontrast CT. (B) Coronal noncontrast CT in the same patient. Ao , Descending thoracic aorta; IVC , inferior vena cava; St , stomach.
- •
The presence of a target sign, indicating submucosal edema, helps to differentiate esophagitis from other causes of wall thickening.
- •
Strictures are seen as areas of luminal narrowing with dilatation of the esophagus above the lesion.
- •
Severe esophagitis may lead to deep ulcers, perforation, mediastinitis, and abscess.
Esophageal Injury and Perforation
Esophageal perforation may be traumatic, may be iatrogenic after instrumentation, or may result from neoplasm or inflammation. Boerhaave syndrome is spontaneous rupture of the esophagus associated with violent vomiting. Because it may be lethal, prompt recognition of esophageal perforation is essential. Underlying esophageal disease is often present.
- •
Wall thickening, high-attenuation intramural hematoma, and mediastinal inflammation are signs of esophageal injury.
- •
Periesophageal fluid or contrast medium and extraluminal mediastinal air are the most specific findings of esophageal perforation ( Fig. 17.10 ).

FIG. 17.10
Perforation of the esophagus.
Perforation of the distal esophagus during a stenting procedure for esophageal stricture is evidenced by extensive air in the mediastinum (straight red arrows) , around the aorta (curved red arrow) , and in the subcutaneous tissues of the chest (red arrowheads) . The esophagus (yellow arrowhead) has a thickened wall. Bilateral pleural effusions (e) are evident.
- •
Pleural effusions are common.
Stomach
Anatomy
The posteriorly located gastric fundus is seen on CT sections through the level of the dome of the diaphragm. The esophagus joins the stomach a short distance below the fundus. A prominent pseudotumor, caused by thickening of the gastric wall as a result of incomplete distention, is often seen near the gastroesophageal junction. Additional distention with more air or contrast agent will eliminate this pseudotumor. The body of the stomach sweeps toward the right. The antrum crosses the midline of the abdomen between the left lobe of the liver and the pancreas to join the duodenal bulb in the region of the gallbladder.
The normal gastric wall should not exceed 5 mm in thickness when the stomach is well distended. Rugal folds are commonly visualized even with good distention. As for the esophagus, benign and malignant conditions produce similar CT findings. CT is performed to document the extent of extraluminal disease.
Technical Considerations
The stomach must be filled with positive contrast medium or distended with air or water for optimal assessment by CT. Oral contrast agent or water (200–300 mL) is routinely given to fill the stomach just before the patient lies down on the CT couch. Alternatively, distention of the stomach with air may be achieved by the giving of gas-producing crystals (4–6 g of citrocarbonate granules with 16–30 mL of water) instead of the opaque contrast agent. A nasogastric tube may also be used to distend the stomach. The patient can be repositioned, in the prone or decubitus position, to optimize distention of the different portions of the stomach with air or contrast agent.
Hiatus Hernia and Gastric Volvulus
Hiatus hernia is a protrusion of any portion of the stomach into the thorax. A major reason to recognize a hiatus hernia is to avoid mistaking it for a tumor. Large hiatus hernias are associated with gastric volvulus.
- •
On CT, a sliding hiatus hernia (95% of hiatus hernias) is identified by recognition of gastric folds appearing above the esophageal hiatus ( Fig. 17.11 ). The herniated stomach may create an air- or contrast-filled mass contiguous with the esophagus above and the remainder of the stomach below.

FIG. 17.11
Hiatus hernia.
Axial CT image showing a portion of the stomach extending through the esophageal hiatus to form a hiatal hernia (red arrowheads) . Gastric folds are evident. Fluid retained within the herniated stomach forms an air-fluid level (yellow arrow) . Ao , Thoracic aorta; Ht , heart; IVC , inferior vena cava.
- •
The edges of the esophageal hiatus are often widely separated, exceeding 15 mm in width.
- •
With a paraesophageal hernia the gastric cardia and gastroesophageal junction are below the esophageal hiatus and the fundus of stomach is above the hiatus adjacent to the distal esophagus. A variant of paraesophageal hernia is coexistence of a sliding hiatal hernia with the paraesophageal intrathoracic fundus.
- •
In organoaxial rotation ( Fig. 17.12 ) the stomach rotates around its long axis, resulting in the convex greater curvature of the stomach being positioned in the chest anteriorly, superiorly, and to the right of the concave lesser curvature.

FIG. 17.12
Paraesophageal hiatus hernia with organoaxial rotation of the stomach.
Axial postcontrast CT shows the stomach (St) in the left chest cavity above the left hemidiaphragm. The stomach has rotated on its long axis so that the greater curvature (red arrow) of the stomach is anterior and to the patient’s right, whereas the lesser curvature (yellow arrow) of the stomach is posterior and to the patient’s left. The gastroesophageal junction (red arrowhead) is just above the esophageal hiatus. A portion of the right hemidiaphragm (Dia) is shown.
- •
In the much less common mesenteroaxial rotation the stomach turns upside down. The antrum and pylorus are superior and in the chest, whereas the fundus is near the diaphragm.
- •
The term gastric volvulus refers to abnormal gastric rotation associated with strangulation and obstruction. Patients have acute abdominal pain, nausea, and vomiting. Complete gastric obstruction occurs with twisting greater than 180 degrees. CT shows gastric distention with retained contrast medium, air, and food. Emergency surgical repair is needed to avoid ischemia.
Thickened Gastric Wall
Thickening of the gastric wall, either focal or diffuse, is an important but nonspecific sign of gastric disease. With good technique, which includes aggressive distention of the stomach with air or contrast agent, wall thickening greater than 5 mm can be considered abnormal. The causes include carcinoma, lymphoma, gastric inflammation (peptic or Crohn disease), perigastric inflammation (pancreatitis), and radiation. CT may show gastric ulcers as collections of contrast agent within a thickened wall. Penetrating ulcers appear as a sinus tract, marked by contrast agent or air, extending to adjacent structures.
Gastritis
Gastritis is a very common disease with numerous causes. Erosive gastritis is caused by nonsteroidal antiinflammatory drugs, aspirin, alcohol, radiation, and ischemia. Nonerosive gastritis is caused most commonly by Helicobacter pylori infection.
- •
Thickened gastric folds are the best CT sign of gastritis. Wall thickening is often focal and most common in the antrum.
- •
When gastritis is erosive, the mucosa may enhance brightly during the arterial phase because of hyperemia, causing a three-layer wall appearance that differentiates this benign condition from malignant wall thickening.
- •
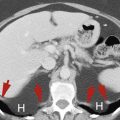
Emphysematous gastritis is a rare life-threatening condition characterized by air within the thickened gastric wall ( Fig. 17.13 ). It is caused by invasion of the gastric wall by gas-producing Escherichia coli or Staphylococcus aureus , accompanying inflammation associated with caustic ingestion or alcohol abuse. The gas may extend into the portal venous system.

FIG. 17.13
Emphysematous gastritis.
CT performed following oral and intravenous contrast medium administration shows gas in the wall (red arrows) of the stomach (St) as well as within the portal vein (yellow arrowhead) in the liver. The wall of the stomach is thickened.
Gastric Carcinoma
Adenocarcinoma is the cause of 95% of gastric malignancy. Risk factors include age of 50 to 70 years, chronic atrophic gastritis, pernicious anemia, familial adenomatous polyposis, and Ménétrier disease. CT is used to stage disease and identify patients whose disease is not surgically resectable.
- •
The primary tumor appears as focal, nodular, or irregular thickening of the gastric wall ( Fig. 17.14 ), or as a polypoid intraluminal mass of soft-tissue attenuation.

FIG. 17.14
Gastric carcinoma.
Nodular thickening of the wall of the distal stomach (St) and gastric antrum (red arrowheads) is striking in comparison with the normal wall of the gastric body (yellow arrow) .
- •
Diffuse wall thickening with irregular narrowing of the lumen is indicative of scirrhous carcinoma (linitis plastica).
- •
Extension of tumor into the perigastric fat is nearly always present when the wall thickness exceeds 2 cm ( Fig. 17.15 ). The serosal surface is blurred, and strands and nodules of tumor are seen in the adjacent fat.

FIG. 17.15
Large gastric carcinoma.
Adenocarcinoma of the distal stomach (St) produces a large heterogeneous mass (Ca) containing low-attenuation areas of necrosis and hemorrhage. The tumor has extended through the stomach wall into the perigastric fat (red arrowhead) , has obliterated the fat plane (red arrow) between the stomach and the pancreas, and has invaded the pancreas (P) . a , Superior mesenteric artery; v , superior mesenteric vein.
- •
Perigastric lymph nodes are considered to be involved when the short axis diameter is more than 6 mm. A round shape and heterogeneous or marked enhancement are additional signs of nodal involvement. Nodes near the celiac axis and in the gastrohepatic ligaments are most likely to be involved.
- •
Hematogenous metastases go first to the liver then to the lungs, adrenal glands, kidneys, bones, and brain.
- •
Peritoneal carcinomatosis may occur.
- •
Local recurrence of gastric carcinoma appears as focal wall thickening at the anastomosis or in the remaining stomach. Nodal recurrence is most common along the course of the hepatic artery or in the para-aortic region. Peritoneal recurrence is seen in the cul-de-sac, on parietal peritoneal surfaces, or on the surface of bowel.
Gastric Lymphoma
The stomach is the most common site of involvement for primary GI lymphoma. Most cases (90%–95%) are non-Hodgkin lymphoma of B-cell origin. Mucosa-associated lymphoid tissue lymphoma is an indolent form of lymphoma with a significantly better prognosis.
- •
Gastric lymphoma may cause a polypoid mass, diffuse wall infiltration with featureless walls, or markedly thickened walls with nodular thickened folds.
- •
CT features that favor lymphoma over carcinoma include more dramatic thickening of the stomach wall (>3 cm), involvement of more than one region of the GI tract, transpyloric spread of tumor (occurs in 30% of gastric lymphoma), and more widespread adenopathy above and below the level of the renal hilum ( Fig. 17.16 ). Luminal narrowing is typical of carcinoma but rare with lymphoma.

FIG. 17.16
Gastric lymphoma.
B-cell lymphoma causes a large mass (between red arrowheads ) that massively thickens the wall of the distal stomach. Compare this with Fig. 17.15 . Note the homogeneous attenuation of lymphoma compared with the large adenocarcinoma. Lymphoma also obliterates the fat plane (red arrow) between the stomach and the pancreas (P) . The spleen (Sp) and two splenules (s) are enlarged.
- •
Low-grade mucosa-associated lymphoid tissue lymphomas are superficial spreading lesions that are seen as mucosal nodularity, shallow ulcers, and minimal fold thickening.
- •
High-grade lymphomas tend to be seen as bulky mass lesions or marked fold and wall thickening.
Gastrointestinal Stromal Tumors
The belief that GI mesenchymal tumors arise from a common precursor cell (the Cajal cell) led to the term gastrointestinal stromal tumor (GIST). Most GISTs arise in the muscularis propria throughout the GI tract, with 60% to 70% of these arising in the stomach and 20% to 30% arising in the small bowel. Lesions are rare in the colon, rectum, and esophagus. Other primary sites of origin include the omentum, mesentery, and retroperitoneum. Gastric tumors previously identified as leiomyomas, leiomyosarcomas, and leiomyoblastomas are now mostly classified as GISTs. Approximately 10% to 30% of GISTs are malignant. GISTs are differentiated from true leiomyomas and leiomyosarcomas by the presence of the protein KIT (CD117), a tyrosine kinase growth factor receptor that is tested for by immunohistochemical stain. Only in the esophagus are leiomyomas more common than GISTs. In other portions of the GI tract, GISTs are the most common mesenchymal tumor. Most tumors present with GI bleeding resulting from mucosal ulceration. GISTs are rarely seen in patients younger than 40 years.
- •
Tumors arise from the bowel wall and grow away from the gut lumen to project into the abdominal cavity. The size ranges from millimeters to 30 cm. Small lesions that are homogeneous in attenuation are usually benign ( Fig. 17.17 ).

FIG. 17.17
Gastric gastrointestinal stromal tumor: benign.
A wall mass (arrowhead) of uniform attenuation and enhancement projects both into the lumen of the stomach (St) and into the abdominal cavity.
- •
Ulceration of the luminal surface is seen in 50% of lesions.
- •
Cystic degeneration, hemorrhage, and necrosis are common especially in large lesions ( Fig. 17.18 ). The tumor cavity may communicate with the gut lumen and may contain air or oral contrast medium. Calcification in the tumor is rare.

FIG. 17.18
Gastric gastrointestinal stromal tumor: malignant.
A huge heterogeneous mass (M) arises from the posterior wall of the stomach (St) . Large low-density areas within the mass correspond to hemorrhage and necrosis. An ulcer crater (arrowhead) is identified within a nodular tumor projection into the gastric lumen.
- •
Contrast enhancement is seen in viable tumor, most commonly in the periphery of the mass.
- •
The risk of malignancy is increased in tumors that arise outside the stomach or are larger than 5 cm. Metastases are most common in the liver and the peritoneal cavity.
Gastric Varices
Gastric varices occur as a result of portal hypertension or splenic vein thrombosis.
- •
Varices appear as well-marginated clusters of rounded and tubular densities in, or adjacent to, the wall of the stomach, most commonly in the fundal region. Bright enhancement with intravenous contrast medium administration clinches the diagnosis ( Fig. 17.19 ).

FIG. 17.19
Gastric varices.
Bolus intravenous contrast medium administration causes bright enhancement of varices (arrowheads) in the wall of the gastric fundus in this patient with alcoholic liver disease and portal hypertension. St , Stomach.
- •
CT signs of liver disease and other portosystemic collateral vessels are often present.
- •
Gastric varices without esophageal varices is a hallmark finding associated with splenic vein thrombosis.
Small Bowel
Anatomy
The duodenum extends from the pylorus to the ligament of Treitz, forming the familiar C loop. The duodenum becomes retroperitoneal at the right free edge of the hepatoduodenal ligament, closely related to the neck of the gallbladder. The descending duodenum passes to the right of the pancreatic head to just below the uncinate process, where the duodenum turns to the left. The horizontal portion crosses anterior to the inferior vena cava and aorta and posterior to the superior mesenteric vein and artery. The fourth portion ascends just left of the aorta to the ligament of Treitz, where the bowel becomes the intraperitoneal jejunum.
The jejunum occupies the left side of the upper abdomen, whereas the ileum lies in the right side of the lower abdomen and right pelvis. Jejunal loops are feathery with distinct folds. Ileal loops are featureless with thin walls. Distention of the lumen with oral contrast medium is essential to adequately evaluate the bowel. Unopacified small bowel may mimic adenopathy and abdominal masses. The small-bowel mesentery contains many vessels that are easily visualized when outlined by fat. The normal luminal diameter of the small bowel does not exceed 2.5 cm. The normal wall thickness is less than 3 mm.
Normal small bowel shows uniform mural enhancement with intravenous contrast medium administration. With full luminal distension the enhancing wall is thin, measuring 1 to 2 mm. Enhancement is best appreciated with the low-attenuation contrast agents routinely used with CT enterography. Absence of mural enhancement is indicative of ischemia. A target appearance of mural enhancement with high-attenuation mucosa and serosa separated by low-attenuation submucosa is indicative of a benign process such as Crohn disease, infection, angioedema, hemorrhage, or radiation enteritis. Heterogeneous enhancement is typical of small-bowel tumors. Mild wall thickening (3–4 mm) is most characteristic of hypoalbuminemia, infectious enteritis, or mild Crohn disease. Moderate wall thickening (5–9 mm) is seen with ischemia caused by mesenteric vein thrombosis, intramural hemorrhage, vasculitis, radiation ( Fig. 17.20 ), and moderate Crohn disease. Marked wall thickening (>10 mm) is associated with lymphoma and other neoplasms, vasculitis, and intramural hemorrhage. Infectious enteritis seldom causes this degree of wall thickening. Mural thickening of more than 20 mm is almost always neoplastic. Benign conditions generally cause symmetric thickening along the circumference of the wall. Asymmetric wall thickening suggests neoplastic disease. Lymphoma is an exception, commonly causing symmetric wall thickening.

Small-Bowel Diverticula
Small-bowel diverticula may cause unusual collections of fluid, air, contrast material, or soft-tissue nodules in the fat and tissues adjacent to the bowel. These must not be mistaken for abscesses, pancreatic pseudocysts, or tumors. Rescanning the patient will often demonstrate a significant change in the appearance of diverticula.
- •
Typically diverticula appear as mucosal sacs without folds and containing air or contrast medium located adjacent to a loop of bowel ( Fig. 17.21 ).