Gastrointestinal Tract
Sudha A. Anupindi
Andria M. Powers
Suma Kannabiran
Jonathan R. Dillman
Michael S. Gee
Asef Khwaja
INTRODUCTION
Pediatric gastrointestinal (GI) disorders include a wide spectrum of entities involving structures from the level of the esophagus to the rectum. Imaging of these structures can provide both anatomic and functional information, which can help managing pediatric patients with congenital and acquired GI disorders. In this chapter, the currently available imaging modalities used to evaluate the pediatric GI tract are reviewed. In addition, various abnormalities of the pediatric GI tract that can be encountered in clinical practice are discussed, including congenital, infectious, inflammatory, and neoplastic processes, focusing on key clinical and imaging features as well as treatment for each entity and providing a differential diagnosis where appropriate.
IMAGING TECHNIQUES
Radiography
Radiography of the chest and/or abdomen (including the pelvis) is the most simple, least expensive, and most widely available imaging examination performed in children presenting with signs and symptoms related to the GI tract, such as dysphagia, chest pain, abdominal pain, or constipation. Although often insensitive, radiographs in some settings can be very specific.
Abdominal radiographs should be appropriately collimated and have a technique (e.g., tube current, tube potential, exposure time) that enables one to clearly visualize the bowel gas pattern, intraperitoneal free air (when present), and viscera. When performing abdominal radiography, two views (commonly supine frontal and cross-table lateral radiographs in young children) are often acquired. Cross-table lateral, decubitus, and upright radiographs of the abdomen help assess for air-fluid levels within the bowel as well as intraperitoneal free air, which can be easily overlooked on supine frontal views. Cross-table lateral radiography is particularly useful in very ill children who cannot tolerate decubitus or upright imaging, with intraperitoneal free air, when present, accumulating anteriorly within the abdomen, often anterior to the liver.
Abdominal radiography is especially helpful when assessing for bowel obstruction, thereby helping localize the obstructing process to the upper or lower GI tract. In the postoperative setting, radiographs can evaluate for a variety of complications as well as provide clues to what surgery has been performed (in the absence of such knowledge). In the setting of suspected or known ingested foreign body, radiographs of the chest and/or abdomen can be obtained to further characterize the ingested object (if radiopaque), establish its location in the GI tract and potential complications, and guide management.
Fluoroscopy
Fluoroscopy is still widely used to assess the pediatric GI tract because of its diagnostic capabilities, availability, and relative low cost compared to computed tomography (CT) and magnetic resonance imaging (MRI). Fluoroscopic studies of the GI tract require administration of an enteric contrast material; common contrast agents used in children include barium, water-soluble iodinated contrast material, and air. Barium can be used to assess most conditions, whereas water-soluble iodinated contrast materials (such as iohexol, a low-osmolality iodinated contrast material that is
commonly administered intravenously for CT, or iothalamate meglumine) are usually employed in cases where bowel perforation is of concern, such as in the setting of acute trauma or the recent postoperative child. Dilute hyperosmolar iodinated contrast materials, such as diatrizoate meglumine and diatrizoate sodium, are less often used in current practice and are reserved for cases where therapeutic cleanout is desired (e.g., in adolescents with cystic fibrosis and distal intestinal obstruction). Hyperosmolar contrast agents have the potential to cause fluid shifts and electrolyte imbalances, and thus should be used with caution. Oral administration of hyperosmolar contrast agents can also cause severe pulmonary complications (e.g., chemical pneumonitis or pulmonary edema) in children if aspirated and should probably be avoided. Instead, a low-osmolality iodinated contrast material should be considered.
commonly administered intravenously for CT, or iothalamate meglumine) are usually employed in cases where bowel perforation is of concern, such as in the setting of acute trauma or the recent postoperative child. Dilute hyperosmolar iodinated contrast materials, such as diatrizoate meglumine and diatrizoate sodium, are less often used in current practice and are reserved for cases where therapeutic cleanout is desired (e.g., in adolescents with cystic fibrosis and distal intestinal obstruction). Hyperosmolar contrast agents have the potential to cause fluid shifts and electrolyte imbalances, and thus should be used with caution. Oral administration of hyperosmolar contrast agents can also cause severe pulmonary complications (e.g., chemical pneumonitis or pulmonary edema) in children if aspirated and should probably be avoided. Instead, a low-osmolality iodinated contrast material should be considered.
In general, low-dose fluoroscopic techniques in accordance with the As Low As Reasonably Achievable (ALARA) principle should always be utilized. This includes (1) using pulse fluoroscopy; (2) maximizing collimation; (3) minimizing magnification; (4) keeping the image intensifier close to the patient; (5) using as little fluoroscopy time as possible to answer the specific clinical question at hand; and (6) minimizing the number of true radiographic exposures and instead using last image capture technique that sufficiently evaluates many conditions.1 With current generation fluoroscopy systems, last image capture technique can also be used to grab multiple images and document dynamic processes, such as GI tract motility.
Esophagography
The esophagram is a focused examination evaluating the anatomy and motility of the esophagus from the level of the hypopharynx through the proximal stomach, including the gastroesophageal (GE) junction. Typical clinical indications for performing esophagography in the pediatric population include evaluation of suspected or known vascular rings and sling, tracheoesophageal fistula (TEF), dysphagia, and foreign body. This study is also frequently used to evaluate the postoperative appearance of the esophagus, most often following TEF (esophageal atresia) repair.
Esophagography is performed by having the patient drink oral contrast material by cup, syringe, or bottle and acquiring fluoroscopic images in the lateral, oblique, and supine positions. When there is high suspicion for N (also known as H)-type TEF, a pull-back esophagram can be performed by placing a feeding tube into the stomach and gradually pulling back the tube and simultaneously injecting contrast material under direct visualization.2 However, care should be taken to not inject contrast material too high within the esophagus or too vigorously, as tracheal aspiration can occur. This can be sometimes mistaken for an N-type TEF. It is important to provide measurements of areas of narrowing, including stricture length and minimum luminal diameter. Occasionally, in older children, swallowed barium tablets may be used to estimate of the size of the esophageal lumen.
Upper Gastrointestinal Series and Small Bowel Follow-Through
Esophagography is often followed by assessment of the stomach and duodenum as part of the same examination; this can be referred to as an upper gastrointestinal (UGI) series. UGI series may also refer to evaluation of the stomach and duodenum when contrast material is delivered directly into the stomach through a gastrostomy tube or nasogastric tube. Typical clinical indications for the UGI series in the pediatric population include the following: (1) vomiting including suspected malrotation with volvulus, gastric outlet obstruction, and duodenal/proximal jejunal obstruction; (2) assessment of upper GI tract anatomy prior to gastrostomy tube placement; and (3) evaluation of gastric emptying and suspected gastroesophageal reflux although other diagnostic tests may be more sensitive for these conditions.
Common UGI series images include the following: (1) supine frontal and left lateral decubitus views of the esophagus; (2) supine and oblique views of the stomach; and (3) right lateral decubitus imaging of the distal stomach and duodenum. Lateral imaging is used to confirm a normal retroperitoneal course of the second through fourth portions of the duodenum, just anterior to the spine. Finally, the child is placed supine to capture the location of the duodenojejunal junction (DJJ), which is normally positioned just to the left of the spine and at the level of the duodenum bulb.
In recent years, although the small bowel follow-through (SBFT) examination largely has been replaced by CT and MRI (cross-sectional enterography), particularly in the evaluation of known or suspected inflammatory bowel disease (IBD), it is still a useful study in the pediatric population. Typical clinical indications for SBFT in children include evaluation of obstruction, feeding intolerance, dysmotility/small bowel transit time, polyposis syndrome, and suspected malabsorption syndrome. This study requires enteric contrast material to be orally ingested by the child or administered through a gastrostomy tube or nasogastric tube. Multiple radiographic images are then acquired (typically at 30- to 60-minute intervals) as contrast material passes through the small bowel until it reaches the proximal colon. Compressive fluoroscopic spot images may also beneficial in some children, especially when there is concern for small bowel polyps or Crohn disease. Occasionally, in the inpatient setting, markedly delayed portable radiographs may provide additional value when assessing dysmotility/small bowel transit time and obstruction.
Contrast Enema
Typical clinical indications for performing a contrast enema in the pediatric population include the following: (1) evaluation of obstruction including distal GI tract obstruction in neonates and post-necrotizing enterocolitis strictures; (2) therapeutic clean-out of colonic fecal material, and (3) ileocolic intussusception reduction. Single-contrast technique is generally sufficient in the large majority of children, with double-contrast technique only very rarely performed. When a colonic polyp (e.g., symptomatic juvenile polyp)
is suspected, assessment may include contrast enema, CT, and/or colonoscopy.3 In the setting of neonatal distal GI tract obstruction, water-soluble iodinated contrast material (such as iothalamate meglumine) is preferred because there is slightly increased risk of bowel perforation. Air is preferred by many institutions for identifying and reducing ileocolic intussusceptions.4
is suspected, assessment may include contrast enema, CT, and/or colonoscopy.3 In the setting of neonatal distal GI tract obstruction, water-soluble iodinated contrast material (such as iothalamate meglumine) is preferred because there is slightly increased risk of bowel perforation. Air is preferred by many institutions for identifying and reducing ileocolic intussusceptions.4
The contrast enema should be started with the child in the left lateral decubitus position with the tip of the catheter in the lower rectum. Contrast material is delivered into the colon via gravity drip. After fluoroscopic imaging the rectum in the lateral position, supine frontal and oblique images are generally acquired along the course of the colon to the level of the cecum and appendix. Reflux of contrast material into the terminal ileum occurs in some children and can help evaluate the distal small bowel. Imaging with compression can sometimes help further characterize focal abnormalities.
Other Special Fluoroscopy Examinations
Fistulography and ostomy studies performed to assess complex GI anatomy (e.g., in the setting of cloacal and anorectal malformations) require careful review of the child’s medical and surgical history to determine the exact imaging procedure to be performed. These studies often serve as a road map for the surgeon prior to definitive surgical treatment. Therefore, it is generally helpful for the radiologist and surgeon to directly communicate prior to the study. These studies are performed using water-soluble iodinated contrast materials such as iothalamate meglumine. When there is concern for a fistula (e.g., between the rectum and genitourinary tract), actual radiographic exposures (in addition to captured images) may provide added value because of increased image detail.
Ultrasound
Ultrasound is a well-established first-line imaging study for evaluation of hypertrophic pyloric stenosis (HPS), appendicitis, and ileocolic intussusception. There is also increasing literature supporting the use of this imaging modality for assessment of children with known or suspected IBD, because it has numerous advantages compared to CT and MRI, including lower cost, no need for sedation/general anesthesia, and no ionizing radiation (when compared specifically to CT).5,6
Linear high-frequency (9 to 18 MHz) transducers provide high-resolution gray-scale images of the pediatric GI tract. Cine, panoramic, and color and power Doppler techniques are also important for assessing the pediatric GI tract. When imaging larger children or when bowel loops are located well posterior to the anterior abdominal wall, lower-frequency curved transducers can be used to improve visualization. Graded compression technique allows improved visualization of both the small and large bowel as well as the appendix by displacing/effacing overlying bowel loops and bringing abnormal bowel closer to the transducer7; abnormal bowel segments (e.g., the appendix when inflamed, terminal ileitis in Crohn disease) are often noncompressible and become readily apparent.
A systematic approach to scanning is helpful when evaluating the bowel (e.g., for intussusception or IBD). When a gastroduodenal abnormality is suspected, the patient can drink water to distend and improve visualization of the stomach and duodenum.5,8 Intravenous ultrasound microbubble contrast agents have been shown to be safe when used in children; however, the availability of contrast-enhanced ultrasound is currently limited in the United States.9
Computed Tomography
Typical clinical reasons for CT imaging of the abdominopelvic GI tract in the pediatric population include the following: bowel and mesenteric injury in the setting of acute trauma, bowel obstruction, tumor involving the bowel, concern for postoperative complication following intestinal surgery, and suspected or known intestinal infection/inflammation. Intravenous and oral contrast materials are often valuable and are typically used by most institutions unless specifically contraindicated. Positive (high attenuation) oral contrast material is appropriate for most indications, with one notable exception being acute trauma where time is critical. In the setting of acute bowel obstruction, children may not tolerate oral contrast material, and this is generally not a major issue as obstructed bowel is frequently already distended with fluid. CT enterography (CTE) requires a neutral (attenuation near water) oral contrast material that allows improved visualization of bowel wall/mucosal postcontrast hyperenhancment. CTE technique in children has been described in multiple publications10,11
Pediatric CT scans of the abdomen and pelvis are typically performed using helical technique in order to acquire an isotropic data set that enables a variety of 2D multiplanar reformations (and 3D reconstructions, if indicated). Similar to fluoroscopy, the ALARA principle should be closely followed when performing pediatric CT. This includes using the lowest tube current (mA) and tube potential (kVp) possible. Recent advances in CT technology, including tube current modulation and iterative image reconstruction techniques, have substantially lowered CT doses while maintaining image quality.12 In special select cases, CT colonography (which includes colonic air insufflation and intravenous contrast material) can be performed safely in children to assess for colonic polyps and masses.3
Magnetic Resonance Imaging
MRI of the GI tract, or MR enterography (MRE), has become a primary imaging modality for evaluating children with known or suspected IBD. MRE can also be used for other indications, such as polyp detection, tumor evaluation, and assessment of non-IBD inflammatory processes.13 Similar to CTE, MRE requires that children drink a substantial volume
of biphasic (T1-weighted hypointense/T2-weighted hyperintense) oral contrast material as well as receive intravenous contrast material (gadolinium chelate). Typical MRI pulse sequences used to image the bowel include single-shot fast spin-echo, balanced steady-state free precession, and post-contrast T1-weighted 3D gradient-recalled echo.13
of biphasic (T1-weighted hypointense/T2-weighted hyperintense) oral contrast material as well as receive intravenous contrast material (gadolinium chelate). Typical MRI pulse sequences used to image the bowel include single-shot fast spin-echo, balanced steady-state free precession, and post-contrast T1-weighted 3D gradient-recalled echo.13
Advantages of MRE versus CTE include lack of ionizing radiation and superior soft tissue contrast resolution. Disadvantages of MRE versus CTE and ultrasound include longer scan times, artifacts due to patient motion/breathing and bowel peristalsis, and the regular need for sedation/general anesthesia. A variety of techniques are available that decrease artifacts related to breathing and motion, including respiratory triggering (or navigator gating) and radial filling of k-space.14 Antispasmolytic medications (e.g., glucagon) can reduce bowel peristalsis.15 Recently, MRI also has been used to image suspected appendicitis as an alternative to CT, especially when ultrasound is nondiagnostic or equivocal. MRI for appendicitis can be performed with or without intravenous contrast material, and it has high sensitivity and specificity.16
Nuclear Medicine
A variety of nuclear medicine studies are currently available and can be used to assess the pediatric GI tract. Radiopharmaceutical doses should be adjusted for patient size (e.g., weight), in accordance with published guidelines. Similar to fluoroscopy and CT, the ALARA principle should be closely followed with patient exposure to radiation kept to a minimum while maintaining diagnostic quality.
Radionuclide Salivagram
The radionuclide salivagram shows the flow of saliva from the mouth to the stomach and can be used to identify tracheal (pulmonary) aspiration. This imaging test is a particularly effective tool for identifying aspiration of oral contents and can provide complementary information to the more frequently performed videofluoroscopic swallowing study.17 During this study, the child is positioned supine and a tiny amount of water (or saline) mixed 99mTc-labeled sulfur colloid is placed in the mouth. Imaging of the neck, chest, and upper abdomen is performed for ˜1 hour. Any radioactivity seen in the lungs, bronchi, or trachea is abnormal and considered evidence of aspiration.
Gastroesophageal Reflux Scintigraphy
Gastroesophageal reflux can be detected with higher sensitivity than UGI series using scintigraphy. During this study, 99mTc-labeled sulfur colloid is delivered into the stomach after the radionuclide is mixed with breast milk, formula, or other meal and ingested. Then, supine imaging of the chest and upper abdomen is performed for ˜30 minutes. Any radioactivity seen in the expected location of the esophagus is considered evidence of gastroesophageal reflux, and the number of episodes can be counted.18,19
Gastric Emptying Scintigraphy
Scintigraphy is considered the gold standard for the assessment of gastric emptying.20 Gastric emptying scintigraphy technique varies across institutions. After an NPO period of several hours, patients are asked to ingest a liquid or solid meal mixed with 99mTc-labeled sulfur colloid. Imaging of the upper abdomen in the left anterior oblique position is performed for ˜90 to 120 minutes. A computer program is used to quantify gastric emptying, including generation of time-radioactivity curves from the region of the stomach and calculation of gastric emptying half-time (time required for 50% of radioactivity to leave the stomach).20
Meckel Scan
Pertechnetate scintigraphy (Meckel scan) is generally considered the test of choice for evaluating the child with unexplained GI tract bleeding due to a suspected Meckel diverticulum. Meckel diverticula are remnants of the omphalomesenteric duct that commonly accumulate 99mTc-pertechnetate because of the presence of gastric mucosa. These structures characteristically appear at the same time gastric mucosa is visualized, and are seen as a focally increased area of radioactivity in the lower abdomen usually in the right lower quadrant. Following the intravenous injection of radionuclide, supine imaging is performed for 30 to 60 minutes. Dynamic and static (including lateral imaging) images are usually acquired. With the advent of single photon emission computed tomography differentiating a true Meckel diverticulum from artifact is possible. The sensitivity of pertechnetate scintigraphy for Meckel diverticulum detection is ˜85%.21
Gastrointestinal Bleeding Scan
GI bleeding scan can be used to evaluate active bleeding from the lower pediatric GI tract. A small aliquot of the child’s own blood is labeled with Tc-99m and reinjected into the patient. A dynamic perfusion study can be performed during the first 1 minute after reinjection of labeled red blood cells, followed by static images every few minutes for 30 to 60 minutes. A positive scan shows abnormal radioactivity emanating from the area of active bleeding conforming to a bowel segment. Delayed imaging also can be performed up to 24 hours after reinjection if initial images are unrevealing and GI tract bleeding continues.19 This test helps by quickly localizing the area of bleeding, which can guide the endoscopist or angiographer to target diagnosis and treatment.
NORMAL ANATOMY
Esophagus
The esophagus is a muscular tube arising from the foregut that extends from the C7 to T10-T11 vertebral levels. The upper one-third contains striated muscle and is innervated by the vagus nerve, and the lower two-thirds contains smooth muscle and is innervated by the splanchnic plexus. The esophagus normally has smooth mucosa and lacks an
outer serosa. There are two esophageal sphincters (upper and lower, respectively); the lower sphincter tends to be immature in infants and contributes to gastroesophageal reflux. The descending thoracic aorta and left mainstem bronchus normally have mild mass effect upon the esophagus that can be appreciated at esophagography. Blood supply to the esophagus is provided by branches of the inferior thyroid, bronchial, left gastric, and left phrenic arteries as well as esophageal branches of the thoracic aorta. Periesophageal and submucosal venous plexi provide venous drainage.
outer serosa. There are two esophageal sphincters (upper and lower, respectively); the lower sphincter tends to be immature in infants and contributes to gastroesophageal reflux. The descending thoracic aorta and left mainstem bronchus normally have mild mass effect upon the esophagus that can be appreciated at esophagography. Blood supply to the esophagus is provided by branches of the inferior thyroid, bronchial, left gastric, and left phrenic arteries as well as esophageal branches of the thoracic aorta. Periesophageal and submucosal venous plexi provide venous drainage.
Stomach
The stomach also arises from the foregut and is made up of five major parts: cardia, fundus, body, antrum, and pylorus. The cardia is a small portion of the stomach located near the gastroesophageal junction. The fundus is the rounded proximal portion of the stomach located below the left hemidiaphragm that also borders the spleen. The body represents the majority of the stomach and is bordered by the lesser (superiorly) and greater (inferiorly) curvatures. The distal portions of the stomach include the antrum and pylorus; the pylorus acting as a sphincter that opens allowing the stomach to empty and closes preventing small bowel contents from reentering the stomach. The stomach is held in place by four major ligaments: gastrophrenic, gastrohepatic, gastrosplenic, and gastrocolic ligaments. Primary blood supply to the stomach is via the left gastric, right gastric, gastroduodenal, and splenic arteries. Primary gastric venous drainage is via the left gastric (coronary), right gastric, and gastroepiploic veins, which all drain to the portal vein. In infants, the stomach can be horizontal, whereas in older children it can assume a J-shape. Prominent folds (rugae) make the stomach easily recognizable on UGI series, CT, and MRI.
Small Intestine
The small intestine is made up of the duodenum, jejunum and ileum. It grows from about 200 cm at birth to a total of about 600 cm (19.8 feet) into adulthood.19 The duodenum is very short in length and is derived from both the foregut and midgut. It has a characteristic c-shape and is composed of four portions. The first portion of the duodenum includes the duodenal bulb and extends from the pylorus to the level of the gallbladder. The second or descending portion of the duodenum is located just lateral to the pancreatic head and includes the major ampulla that drains pancreaticobiliary ducts. The third or horizontal portion of the duodenum normally crosses from right to left just anterior to the spine, whereas the fourth or ascending portion extends to the DJJ, where the small bowel should be fixed by the ligament of Treitz. The second through fourth portions of the duodenum should be retroperitoneal in location. Primary blood supply to the duodenum is via the gastroduodenal and pancreaticoduodenal arteries.
The jejunum makes up about 40% of the small bowel, whereas the more distal ileum composes the remainder. The jejunum is most often located in the left upper quadrant of the abdomen, although in some children it may reside in the right upper quadrant (this is normal as long as the ligament of Treitz is appropriately located). The jejunum has visible circular folds, so-called valvulae conniventes, which often have a feathery appearance at ultrasound, SBFT, CT, and MRI. At CT and MRI, it is common for the jejunum to enhance more than ileum. The ileum is primarily located in the lower abdomen and pelvis before ending in the right lower quadrant as the terminal ileum. Normal ileum appears relatively featureless. The primary blood supply to the small bowel is via the superior mesenteric artery (SMA). Mesenteric arterial branches anastomose to form a complex vascular arcade that eventually terminates as the vasa recta. Small bowel venous drainage is via portal venous system (superior mesenteric vein).
Colon
The colon arises from both the midgut and hindgut, and it has multiple segments, including cecum, ascending colon, transverse, descending colon, and sigmoid colon. It grows from about 30 to 40 cm at birth to about 150 cm by adulthood.19 The most proximal portion of the colon, the cecum, is normally located in the right lower quadrant and may be mobile in infants. The appendix arises from the cecum, varies in length, and blind ends. Three longitudinal bands of smooth muscle (taeniae coli) extend from the cecum to the sigmoid colon and are responsible for colonic sacculations called haustra. Numerous peritoneum-lined fat and blood vessel-containing pouches called epiploic appendages line much of the colon. The colon takes two 90 degree bends called flexures, the hepatic flexure in the right upper quadrant and the splenic flexure in the left upper quadrant. The ascending and descending portions of the colon are retroperitoneal in location, whereas the appendix, cecum, transverse, and sigmoid colon are intraperitoneal and have mesenteries. Blood supply to the colon is provided by the right and middle colic branches of the SMA as well as by the left colic artery, which arises from the inferior mesenteric artery (IMA). Venous drainage is via the portal venous system (superior and inferior mesenteric veins).
Rectum
The rectum is vertically oriented with the rectosigmoid junction located at approximately the level of the sacral promontory. The rectum subsequently gives rise to the anal canal about 4 cm from the anal verge.22 Importantly, the dentate line is located within the anal canal and this undulating demarcation marks the transition from columnar to squamous epithelium.23 The distal rectum and anal canal are extraperitoneal in location and bordered laterally by the ischiorectal fossae.22 Rectal blood supply is via the superior rectal artery
arising from the IMA and middle rectal artery arising from the internal iliac artery. Venous drainage above the dentate line is via the portal system, whereas venous drainage below the dentate line is via systemic veins.22
arising from the IMA and middle rectal artery arising from the internal iliac artery. Venous drainage above the dentate line is via the portal system, whereas venous drainage below the dentate line is via systemic veins.22
SPECTRUM OF GASTROINTESTINAL TRACT DISORDERS
Congenital and Developmental Anomalies
Esophagus
Esophageal Atresia and Tracheoesophageal Fistula
Esophageal atresia is part of a spectrum of anomalies involving the foregut that can occur either in isolation or in association with TEF. The reported incidence of esophageal atresia is about 1:3,500 births, with a slight male predominance.24 The exact underlying cause is currently unclear, although it is thought to be due to abnormal formation and separation of primitive foregut into the esophagus and trachea,19 perhaps because of a vascular insult. There are four types of TEF, the most common being proximal esophageal atresia with an associated fistula between the trachea and distal esophagus, occurring in nearly 85% of cases (Table 16.1). Esophageal atresia/TEF sometimes occurs in conjunction with other anomalies, such as DiGeorge syndrome or as a part of the VACTERL association (vertebral, anorectal, cardiac, tracheoesophageal, renal, and limb anomalies).
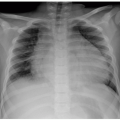
Prenatal imaging may suggest esophageal atresia based on polyhydramnios and nonvisualization of the stomach, although some affected pediatric patients present initially in the neonatal period. Presenting symptoms after birth include difficulty handling secretions, regurgitation, choking, respiratory distress, and recurrent pneumonia especially in the setting of H or N-type TEF. Radiography often reveals a distended air-filled blind-ending proximal esophagus. In addition, a nasogastric tube may be seen coiled in the proximal esophageal pouch. The presence of bowel gas confirms the presence of a TEF (Fig. 16.1). Fluoroscopic pouchogram performed by instilling a very small volume of contrast material into the proximal esophagus through an end-hole catheter can confirm esophageal atresia. It may also help to identify the presence of a proximal TEF. In addition, esophagram can be performed to identify a suspected H or N-type TEF (Fig. 16.2).
TABLE 16.1 Classification of Tracheoesophageal Fistulas | ||
|---|---|---|
|
The current treatment is surgical, with most affected pediatric patients undergoing primary esophageal repair and TEF takedown. Gastric pull-through may be required in children
with long-gap esophageal atresia. Prognosis depends on the length of the atretic esophageal segment and presence of other congenital abnormalities.25 Anastomotic esophageal strictures are common and can be readily identified by esophagography.
with long-gap esophageal atresia. Prognosis depends on the length of the atretic esophageal segment and presence of other congenital abnormalities.25 Anastomotic esophageal strictures are common and can be readily identified by esophagography.
Esophageal Duplication Cyst
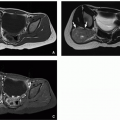
Esophageal duplication cysts are the second most common GI tract duplication anomaly, after ileal duplication cysts.26 These cysts most often arise from the mid- to lower portion of the esophagus26 and contain foregut-derived tissues. Esophageal duplication anomalies are most often cystic, presenting as eccentric round or spherical (and less often tubular) lesions in or adjacent to the esophageal wall.27 Histologically, these lesions typically have a muscular wall and can be lined by a variety of types of epithelium.28 Although commonly asymptomatic and incidentally detected, some affected children may report dysphagia or other symptoms including chest pain and respiratory distress.
At radiography, esophageal duplication cysts either are imperceptible or appear as middle or posterior mediastinal masses. Esophagography may reveal an intramural mass or extrinsic mass effect upon the esophagus; these anomalies rarely communicate with the esophageal lumen. If visible at ultrasound, esophageal duplication cysts appear as discrete cystic masses with a layered wall.19 At CT, they are circumscribed, homogeneous, near-water attenuation lesions lacking postcontrast enhancement associated with the esophageal wall27 (Fig. 16.3). At MRI, they are usually thin-walled, hypointense on T1-weighted sequences and hyperintense on T2-weighted sequences, unless complicated.
Treatment, especially if symptomatic, is surgical resection.
Stomach
Agastria/Microgastria
Complete absence of the stomach, known as agastria, is extremely rare.27 Such anomalies are frequently associated with other anomalies, including heterotaxy syndromes. Radiography can demonstrate a dilated air-filled esophagus, and the stomach bubble may or may not be seen. Microgastria is a rare developmental anomaly in which the stomach is diminutive, often having a dysmorphic tubular shape (Fig. 16.4). In the setting of microgastria, upper GI series shows a small, sometimes midline stomach with associated esophageal dilatation secondary to poor stomach capacity (Fig. 16.5). Gastroesophageal reflux is common and severe in affected pediatric patients.
Gastric Duplication Cyst
Gastric duplication cysts are rare, accounting for <10% of GI tract duplications.19 These cysts are more common in girls, and most are located along the greater curvature.27 Gastric
duplications are most often asymptomatic, although some affected children may present with obstructive symptoms if large or located near the gastric outlet. Uncommonly, gastric duplications can be associated with ectopic pancreatic tissue leading to pancreatitis, and they can mimic other fluid collections, such as pancreatic pseudocysts.29
duplications are most often asymptomatic, although some affected children may present with obstructive symptoms if large or located near the gastric outlet. Uncommonly, gastric duplications can be associated with ectopic pancreatic tissue leading to pancreatitis, and they can mimic other fluid collections, such as pancreatic pseudocysts.29
 FIGURE 16.5 Microgastria in a neonatal boy. Upper GI series image shows an abnormally small stomach with tubular morphology (arrows). |
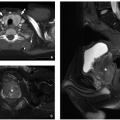
Radiography may demonstrate mass-like soft tissue opacity in the gastric or perigastric region. These lesions are more likely to be seen by ultrasound, appearing as circumscribed anechoic or complicated cystic mass with a layered wall because of underlying echogenic mucosa and hypoechoic muscularis.19,27 At CT, gastric duplication cysts appear as well-circumscribed, homogeneous, low-attenuation masses associated with the stomach wall that lack post-contrast enhancement. At MRI, they are usually thin-walled, T1-weighted hypointense, and T2-weighted hyperintense, unless complicated (Fig. 16.6).19
The current treatment is surgical resection, especially if symptomatic.
Gastric Volvulus
Gastric volvulus refers to abnormal twisting of the stomach that can be either organoaxial (along its long axis) or mesenteroaxial (along its short axis). Gastric volvulus may be idiopathic (related to incomplete fixation of the stomach due to absent or lax ligaments) or it can occur secondary to paraesophageal hiatal hernia, congenital diaphragmatic hernia/eventration of the diaphragm, or wandering spleen.30 In organoaxial volvulus, the greater curvature is located above the lesser curvature19 (Fig. 16.7). In mesenteroaxial volvulus, the pylorus and antrum can be located superior to the proximal stomach. Presentation of gastric volvulus can be acute or chronic.19 Acutely, affected children present with abdominal pain and gastric outlet obstruction, including persistent vomiting. Chronic gastric volvulus may manifest as intermittent abdominal pain or vomiting. Ischemia can develop if volvulus persists. Radiography can suggest this diagnosis, showing severe gastric distension with little to no distal bowel gas.19 Other features include abnormal gastric morphology, two air-fluid gastric levels (mesenteroaxial), convex superior contour of the gastric body (organoaxial), or visualization of the pylorus at or above the level of the gastric fundus (mesenteroaxial). Upper GI series can confirm the diagnosis, including the exact type of volvulus.30
The current treatment of gastric volvulus is surgical gastropexy.
Antral Web
Antral webs are a cause of partial gastric outlet obstruction and gastroesophageal reflux.27 In this entity, a thin diaphragm or membrane circumferentially lines the gastric antrum, usually within 2 cm of the pylorus. The web is composed of mucosa and submucosa.19 Radiography may reveal proximal gastric distention. Upper GI series shows a thin linear circumferential filling defect involving the distal stomach (Fig. 16.8). It is important to understand that early and careful gastric imaging is a key because the web can be obscured by contrast material.
The symptomatic antral web requires surgical antroplasty or web excision with or without pyloroplasty.
Hiatal Hernia
Hiatal hernia is diagnosed when a portion of the stomach extends through the diaphragmatic hiatus into the thorax. The hernia can be sliding (Type I), in which the gastroesophageal junction is located above the diaphragm, or paraesophageal (Type II), in which the gastroesophageal junction remains below the diaphragm but a portion of the stomach herniates into the thorax. Type III hiatal hernia has both sliding and paraesophageal components, whereas Type IV is diagnosed when other abdominal contents herniate into the chest. Sliding hiatal hernias are most common and make up over 95%.31 This condition is due to widening of the diaphragmatic hiatus and weakening of the phrenicoesophageal membrane.31 When symptomatic, sliding hiatal hernias tend to present with gastroesophageal reflux. Paraesophageal hernias can present with vomiting, dysphagia, and more serious complications, such as gastric obstruction or volvulus.32
 FIGURE 16.8 Antral web in a 4-year-old boy. Spot film from an upper GI series outlines a linear filling defect (arrow) in gastric antrum representing the web. |
Radiographs are commonly normal, although the gastric air bubble or an air-fluid level may be observed in the lower posterior mediastinum.31 Fluoroscopic imaging (upper GI series or esophagography) can confirm the locations of the gastroesophageal junction and stomach as well as characterize the type of hiatal hernia (Fig. 16.9).31
Treatment of sliding and paraesophageal hiatal hernias is surgical, and can include Nissen fundoplication
(a procedure in which the gastric fundus is partially or completely wrapped around the distal esophagus to reestablish competency at the gastroesophageal sphincter33). Post-Nissen fundoplication imaging should show smooth narrowing (about 2 to 3 cm) of the distal esophagus (at the location of the wrap), and the gastric fundus should be located below the diaphragm.33 The complications of Nissen fundoplication are important to recognize which include esophageal obstruction from an overly tight wrap, wrap migration above the diaphragm, and wrap loosening33 (Fig. 16.10).
(a procedure in which the gastric fundus is partially or completely wrapped around the distal esophagus to reestablish competency at the gastroesophageal sphincter33). Post-Nissen fundoplication imaging should show smooth narrowing (about 2 to 3 cm) of the distal esophagus (at the location of the wrap), and the gastric fundus should be located below the diaphragm.33 The complications of Nissen fundoplication are important to recognize which include esophageal obstruction from an overly tight wrap, wrap migration above the diaphragm, and wrap loosening33 (Fig. 16.10).
Small Intestine
Duodenal and Other Small Bowel Atresias
Small bowel atresias are a common source of neonatal intestinal obstruction, with their clinical and radiologic presentations varying depending on location. Duodenal atresia is due to failed intestinal recanalization in utero, is most often preampullary, and often occurs in association with other anomalies,34 including trisomy 21 and VACTERL association (including esophageal atresia). Concurrent malrotation and annular pancreas also have been described. Fetal imaging (ultrasound and MRI) may suggest duodenal atresia based on polyhydramnios and the presence of dilated fluid-filled stomach and duodenum (“double bubble” sign) (Fig. 16.11). Affected neonates present very early in life with vomiting and marked gastric distention. Radiography shows proximal GI tract obstruction, often having the characteristic “double bubble” appearance due to gaseous distention of the stomach and duodenum34; no distal bowel gas should be present except in very rare cases where gas may be seen distally because of passage through pancreatic ducts. Upper GI series can be used to confirm the diagnosis, if necessary. Treatment is surgical, most often duodenoduodenostomy.
Jejunal and ileal atresias are other causes of neonatal intestinal obstruction that are more common than duodenal atresia.19 Such intestinal atresias may be due to intrauterine disruption of blood flow, and there is an association with cystic fibrosis. Uncommonly, intestinal atresias may be both multiple and hereditary.35 Jejunal atresias are most often proximal, whereas ileal atresias are most often distal in location. Jejunal and ileal atresias can be associated with other congenital anomalies, but are not associated with trisomy
21. Affected pediatric patients typically present with vomiting (possibly bilious) and abdominal distention. Pathologic examination shows one or more areas of absent small bowel lumen (Fig. 16.12).
Radiography reveals evidence of proximal GI tract obstruction in the setting of jejunal atresia (few dilated bowel loops) (Fig. 16.13), whereas ileal atresia appears as a distal GI tract obstruction (numerous dilated bowel loops) (Fig. 16.14).34 Gaseous distention of the stomach, duodenum, and proximal jejunum, the “triple bubble” sign, is characteristic of proximal jejunal atresia34 (Fig. 16.13). Upper GI series can be used to confirm suspected jejunal atresia (Fig. 16.13). When ileal atresia is suspected, contrast enema is usually performed. Imaging findings consistent with ileal atresia include an unused microcolon and nondilated small bowel loops distal to the site of atresia34 (Fig. 16.14).
All intestinal atresias are treated surgically, with the exact surgical approach depending on the location of the involved bowel segment, number of intestinal atresias, and association with other abnormalities, such as malrotation.
Duodenal Stenosis and Web
Congenital duodenal stenosis is a cause of partial proximal small bowel obstruction and is thought to be due to abnormal intestinal recanalization in utero. Similar to duodenal atresia, there is an association with other congenital anomalies, including trisomy 21. Duodenal stenosis is associated with annular pancreas or malrotation in up to one-third of affected children.19 Clinical presentation can be because of signs and symptoms of partial small bowel obstruction or related to associated anomalies if the stenosis is mild.
Radiography can show findings similar to duodenal atresia, although bowel gas is generally seen distal to the duodenum (Fig. 16.15). Upper GI series reveals contrast in dilated stomach and proximal duodenum, with eventual passage of contrast material through the area of narrowed duodenum into more distal small bowel.27 A duodenal web is a thin
obstructing membrane with a central aperture within the duodenum that can appear as a filling defect or “windsock” when surrounded by contrast material (Fig. 16.16).
obstructing membrane with a central aperture within the duodenum that can appear as a filling defect or “windsock” when surrounded by contrast material (Fig. 16.16).
Symptomatic duodenal stenoses and webs are most often treated surgically.
Malrotation and Midgut Volvulus
Malrotation refers to abnormal in utero rotation of the bowel with associated mesenteric bands and abnormal mesenteric fixation that can lead to volvulus and subsequent intestinal ischemia and infarction. Numerous anomalies are associated with malrotation, including chromosomal abnormalities. Malrotation in the absence of volvulus is commonly asymptomatic, whereas neonates with volvulus generally present with bilious emesis due to proximal small bowel obstruction.19,36 Older children with malrotation can present with sudden abdominal pain and vomiting due to acute obstruction19 or have milder intermittent symptoms if the bowel is only intermittently twisting and untwisting.
Radiography can be normal, and on occasion may show evidence of proximal small bowel obstruction. Careful evaluation of the bowel gas pattern may reveal abnormal location of the jejunum and proximal colon. The upper GI series is the diagnostic test of choice to evaluate for malrotation. Frontal imaging should show abnormal location of the DJJ (ligament of Treitz), which is normally located at the level of the duodenal bulb and lateral to the left vertebral pedicle
(Fig. 16.17). When midgut volvulus is concomitantly present, the proximal duodenum may be dilated and abruptly cut-off, similar to duodenal atresia. The duodenum can also have a “corkscrew” appearance in affected children (Fig. 16.18). Lateral imaging should be performed in order to confirm that the duodenal course is abnormal and that there is lack of appropriate duodenal retroperitoneal fixation. When upper GI series findings are equivocal, documentation of position of the cecum can be useful for estimating the length of the small bowel mesentery.
(Fig. 16.17). When midgut volvulus is concomitantly present, the proximal duodenum may be dilated and abruptly cut-off, similar to duodenal atresia. The duodenum can also have a “corkscrew” appearance in affected children (Fig. 16.18). Lateral imaging should be performed in order to confirm that the duodenal course is abnormal and that there is lack of appropriate duodenal retroperitoneal fixation. When upper GI series findings are equivocal, documentation of position of the cecum can be useful for estimating the length of the small bowel mesentery.
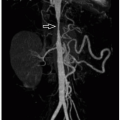
Ultrasound also may be used to diagnose malrotation in children. The normal duodenum should be seen taking a retroperitoneal course, passing between the abdominal aorta and proximal SMA.37 Ultrasound also can evaluate the relationship of the SMA to the superior mesenteric vein; abnormal swirling (“whirlpool” sign) of these vessels can be seen in the setting of malrotation with volvulus (Fig. 16.18). CT typically is not used to evaluate suspected malrotation or midgut volvulus, although on occasion it can show this diagnosis in children presenting with abdominal pain. Also, CT performed for nonemergent indications may occasionally and incidentally reveal malrotation.
Malrotation is treated surgically with the Ladd’s procedure, which consists of untwisting of volvulus (if present), placing the bowel in a nonrotated state to widen the small bowel mesentery, division of any congenital (Ladd’s) bands, and appendectomy.
Meconium Ileus
Meconium ileus is a form of neonatal distal small bowel obstruction due to abnormal obstructing viscous, tenacious meconium. This condition is responsible for ˜20% of neonatal bowel obstructions and is almost always due to underlying cystic fibrosis.34 Approximately 15% to 20% of children with cystic fibrosis present with meconium ileus. In utero, meconium ileus can cause bowel perforation with meconium peritonitis, resulting in peritoneal calcifications and meconium pseudocyst formation.19 Affected infants usually present with failure to pass meconium and symptoms of bowel obstruction, including vomiting and abdominal distension.
Radiography usually shows multiple dilated loops of bowel, consistent with distal GI tract obstruction.38 Retained meconium in right lower quadrant distal small bowel loops may have a “soap bubble” appearance. If prenatal perforation occurred, peritoneal calcifications can be identified and mass effect can be seen on occasion because of large pseudocysts. Contrast enema findings of meconium ileus include abnormally small caliber of the colon (microcolon) as well as the presence of numerous filling defects in the distal small bowel due to meconium19 (Fig. 16.19). In some affected children, it is possible to reflux contrast material into dilated small bowel loops located proximal to the site of obstruction. Contrast enema should not be performed on neonates with evidence of complicated meconium ileus (e.g., acute perforation), because this requires urgent surgery.19 Ultrasound, in addition to radiography, can be used to detect meconium pseudocysts, which often appear as circumscribed round or ovoid cystic lesions with an echogenic rim due to calcification.19 These collections can be located throughout the peritoneal cavity as well as in the inguinal canal and scrotum of boys (Fig. 16.20).
Simple and uncomplicated meconium ileus often can be successfully treated conservatively with water-soluble contrast enemas as well as fluid and electrolyte monitoring and replacement.39 Operative treatments, such as enterostomy and decompression, resection and stoma formation, or resection and anastomosis, may be needed when the nonoperative treatment fails.
Small Bowel Duplication Cysts
Most small bowel duplications cysts involve the terminal ileum and fail to communicate with the bowel lumen. Like GI tract duplication cysts located elsewhere, these small bowel duplication cysts have a muscular wall and can be lined by a variety of types of epithelium. They are typically located along the antimesenteric border of the affected small bowel segment.28 Small bowel duplications are most often asymptomatic, although affected patients can present with abdominal pain or obstructive symptoms because of mass effect or intussusception with the cyst serving as a lead point. Symptoms can also be due to heterotopic mucosa (e.g., gastric mucosa), which may cause GI bleeding.
Most small bowel duplication cysts cannot be seen by radiography, although large cysts may show mass effect appearing as a soft tissue mass (Fig. 16.21). Fluoroscopic studies may show a luminal contour abnormality compatible with an extrinsic mass or less likely obstruction because of mass effect or intussusception. Enteric duplication cysts are most often seen by cross-sectional imaging, appearing as round/ovoid cystic masses associated with bowel wall/mesentery at ultrasound. The wall of these lesions typically has a layered appearance at ultrasound due to hypoechoic muscle and hyperechoic mucosa. At CT and MRI, enteric duplication cysts appear as well-circumscribed, nonenhancing cysts associated with the bowel wall/mesentery (Fig. 16.21). These lesions may appear complicated (thick-walled, attenuation greater than water at CT, increased T1-weighted and decreased T2-wegihted signal at MRI) if they are associated with hemorrhage or infection.
The current treatment is surgically resection, especially if symptomatic.
Meckel Diverticulum
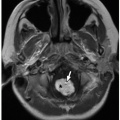
Meckel diverticula are remnants of the omphalomesenteric duct, arising from the antimesenteric border of the distal ileum (Fig. 16.22).19 The remnant can have a persistent patent or fibrous connection to the umbilicus. Most Meckel diverticula are asymptomatic; however, when symptomatic, most affected children present with painless GI tract bleeding due to heterotopic gastric or pancreatic mucosa within the diverticulum. Inflammation of the diverticulum due to luminal obstruction can clinically and radiologically mimic appendicitis. Small bowel obstruction can occur because of inversion of the diverticulum into the ileal lumen with resultant intussusception or small bowel volvulus around the umbilical connection.
 FIGURE 16.22 Meckel diverticulum. Meckel diverticulum, projecting from the antimesenteric aspect of the ileum in a 16-year-old boy whose history included abdominal pain. |
Meckel diverticula are not usually seen at radiography, although related complications, such as bowel obstruction, may be visualized. Associated enteroliths may also be seen. At fluoroscopic imaging, Meckel diverticula appear as saccular outpouchings from the antimesenteric border of the distal ileum.21 At ultrasound, CT, and MRI, Meckel diverticula may have an appearance similar to the appendix. These modalities can also identify diverticulum-related inflammation (diverticulitis) and bowel obstruction. The customary imaging examination used to identify Meckel diverticula is the Meckel’s scan (99mTc-pertechnetate scintigraphy). When a Meckel diverticulum that contains gastric mucosa is present, the diverticulum is typically seen as focally increased radiotracer activity in the right mid- to lower abdomen that appears at the same time as the stomach (Fig. 16.23). Meckel’s scan is ˜90% sensitive and 95% specific for accurately detecting Meckel diverticula that contain gastric mucosa.21
Treatment is surgical resection of the Meckel diverticulum.
Intestinal Lymphangiectasia
Congenital intestinal lymphangiectasia is a primary lymphangiectasia, referring to congenital obstruction and dilatation of the lymphatic drainage of the bowel. Affected pediatric patients may present with soft tissue edema, protein-losing enteropathy, ascites, pleural effusion, and poor weight gain.40 Imaging features reflect lymphatic congestion and leakage, with SBFT, CT, and MRI demonstrating nodular wall/fold thickening of the jejunum and ileum41 (Fig. 16.24). Treatment is primarily a low-fat diet with medium-chain triglyceride supplementation.40
Colon
Colonic Atresia
Colonic atresia is the least common intestinal atresia, and it can be associated with other congenital anomalies, including Hirschsprung disease. In 15% to 20% of cases, there are other areas of intestinal atresia.19 Colonic atresia, like jejunal and ileal atresia, is thought to be the result of intrauterine disruption of blood supply. Affected pediatric patients typically present during the neonatal period with lower GI tract obstruction symptoms, including vomiting, abdominal distension, and failure to pass meconium.
Radiography typically shows distal GI tract obstruction, with numerous dilated loops of bowel.42 Contrast enema reveals a small caliber colon to the point of atresia, which can be located anywhere along the length of the colon, where the contrast column abruptly terminates, without intraluminal filling defects (Fig. 16.25).
Colonic atresia requires surgical management, commonly including an initial diversion operation.
Colonic Duplication Cysts
Large bowel duplication cysts are less common than small bowel duplication cysts and can be classified as appendiceal, colonic, or colorectal tubular duplications.28 The proximal colon (cecum) is a common location. Although often asymptomatic, colonic duplications can present with bowel obstruction due to the cyst serving as a lead point for intussusception, bowel obstruction from mass effect, or GI tract bleeding due to ectopic gastric mucosa. Colorectal tubular duplications are tubular structures neighboring the involved colon that may or may not communicate with the true colonic lumen (Fig. 16.26). Colorectal tubular duplications can terminate blindly, terminate at a separate perineal anal orifice, or terminate via a fistula to the genitourinary tract.43
At ultrasound, colonic duplication cysts are associated with the wall of the colon and appear similar to GI tract duplication cysts located elsewhere, having a layered appearance (Fig. 16.27). CT and MRI demonstrate a well-defined, nonenhancing cystic mass abutting the colon; a communication between the duplication cyst and true colonic lumen may or may not be seen (Fig. 16.27). Also, like duplication cysts elsewhere, colonic duplication cysts on occasion may appear complicated because of internal bleeding or infection. Contrast enemas may reveal extrinsic mass effect on the colon or opacification of the lesion if it communicates with the true lumen of the colon.
Treatment of symptomatic pediatric patients with colonic duplication cysts is surgical resection.
Meconium Plug Syndrome (also known as Small Left Colon Syndrome or Functional Immaturity of the Colon)
Functional obstruction of the distal colon in neonates is called by a variety of names, including meconium plug syndrome, and is likely due to colonic dysmotility.38 This condition is thought to be due to immature bowel peristalsis and abnormal water reabsorption from the colon.19 Meconium plug syndrome, in particular, is used to describe a small caliber descending colon in association with retained colonic meconium. Infants of diabetic mothers and very-low-birth-weight infants are at increased risk. Affected infants typically present with evidence of distal bowel obstruction, including vomiting and abdominal distension.38
Radiography shows a distal GI tract obstruction with multiple dilated loops of bowel. At contrast enema, the distal colon (descending and sigmoid portions) appear smaller in caliber than expected, and a long luminal filling defect consistent with a meconium plug may be seen (Fig. 16.28). Water-soluble contrast enemas can be both diagnostic and therapeutic.
Rectal biopsy is commonly performed after relief of obstruction to exclude Hirschsprung disease.44
Hirschsprung Disease
Hirschsprung disease refers to aganglionosis of the colon, which is more often partial than complete. In this condition, abnormal migration of vagal neural crest cells results in
lack of intramural ganglion cells (extending from the pectinate line a variable length proximally) and functional bowel obstruction (Fig. 16.29).19 Hirschsprung disease is associated with multiple congenital anomalies and syndromes, including trisomy 21. Affected neonates typically present with evidence of distal bowel obstruction, including vomiting and abdominal distension. Hirschsprung disease can also present in older children with severe chronic constipation.
lack of intramural ganglion cells (extending from the pectinate line a variable length proximally) and functional bowel obstruction (Fig. 16.29).19 Hirschsprung disease is associated with multiple congenital anomalies and syndromes, including trisomy 21. Affected neonates typically present with evidence of distal bowel obstruction, including vomiting and abdominal distension. Hirschsprung disease can also present in older children with severe chronic constipation.
In neonates, radiography shows evidence of distal GI tract obstruction with multiple dilated bowel loops and air-fluid levels.38 When Hirschsprung disease is suspected, contrast enema is usually the initial imaging test of choice. Care should be taken to place the enema catheter in the very distal rectum so that the entire rectum can be evaluated. Fluoroscopic imaging is initially performed in the lateral position in order to assess the caliber of the rectum compared to the sigmoid colon. In Hirschsprung disease, the distal aganglionic colonic segment is usually narrowed, and there is dilatation of proximal normal bowel (the rectosigmoid ratio is <1); a distinct “transition zone” strongly supports a diagnosis of Hirschsprung disease (Fig. 16.30). The wall of the abnormal colonic segment may appear irregular having a “saw tooth” appearance. In the setting of total colonic aganglionosis, which can extend to involve the small intestine, the colon can appear normal or may be diffusely small in caliber (i.e., diffuse microcolon). In affected older children who present with chronic constipation, radiography typically shows very large amounts of colonic fecal material with or without colonic dilatation.
Rectal biopsy is performed to confirm the diagnosis of Hirschsprung disease, and treatment is surgical resection of the aganglionic colon.
Megacystis-Microcolon-Intestinal Hypoperistalsis Syndrome
Megacystis-microcolon-intestinal hypoperistalsis (MMIH) syndrome, or Berdon syndrome, is a rare constellation of features, including functional bowel obstruction, marked enlargement of the urinary bladder, microcolon, and malrotation.45,46 This condition is mostly affects girls, and it shares some radiologic features with prune-belly syndrome.47 Clinically, affected children present with functional bowel obstruction, decreased bowel sounds, and abdominal distention.45
Radiography in the neonate commonly reveals dilated small bowel loops, whereas upper GI series shows malrotation. SBFT examination typically shows diffusely dilated, markedly hypoperistaltic small bowel loops and delayed transit of contrast material to the colon. Contrast enema demonstrates
diffuse microcolon with abnormal location of the cecum. Ultrasound in the neonate reveals marked distention of the urinary bladder and possible hydroureteronephrosis.45 MMIH syndrome can be suspected based on prenatal imaging because of the presence of megacystis and dilated small bowel loops (Fig. 16.31).
diffuse microcolon with abnormal location of the cecum. Ultrasound in the neonate reveals marked distention of the urinary bladder and possible hydroureteronephrosis.45 MMIH syndrome can be suspected based on prenatal imaging because of the presence of megacystis and dilated small bowel loops (Fig. 16.31).
This condition has typically had a dismal prognosis, with described management including total parenteral nutrition and bowel transplantation.47
Rectum
Anorectal Malformations
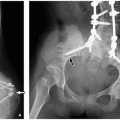
Anorectal malformations represent a spectrum of anomalies involving the rectum and anus, including imperforate anus, anal atresia and stenosis, and rectal atresia. Anorectal malformations frequently coexist with rectourethral fistulas in boys or rectovaginal fistulas in girls. These malformations can be associated with other anomalies, most notably the VACTERL association. Both sporadic and inherited cases occur.48 Currarino syndrome, which includes anorectal malformation, presacral mass (teratoma or anterior meningocele), and malformed sacrum, is autosomal dominantly inherited.49 Most anorectal malformations are apparent at birth, except for rectal atresia, which can manifest as distal bowel obstruction. Fistulas may be clinically apparent because of identification of fecal material in the urethra or vagina. Anorectal malformations have been described as high, intermediate, or low in relation to the levator ani muscles.19 A separate system described by Levitt and Pena classifies these malformations based on the gender of the child and the particular components of the abnormality to provide better prognostic and treatment information.50
The role of imaging in anorectal anomalies is to depict relevant anatomy, detect fistula tracts, and identify associated congenital anomalies. Imaging also helps with surgical planning and helps predict prognosis. The imaging appearances of anorectal malformations vary depending on the exact anomaly.48 Abdominal radiography may show evidence of distal bowel obstruction as well as commonly an anomalous sacrum (Fig. 16.32). Cross-table lateral imaging with the patient prone can be performed to estimate the distance between the lower rectum and skin surface in the setting of imperforate anus. Voiding cystourethrography and augmented-pressure colostogram can be performed to detect and characterize fistulas (Fig. 16.33).51 Ultrasound also can be performed to evaluate the distal rectum using a transanal approach, especially in the setting of imperforate anus. Currently, MRI is being increasing used in both the preoperative and postoperative settings to characterize pelvic floor musculature, search for fistulas, and identify associated anomalies48 (Fig. 16.33).
Treatment of anorectal malformations is surgical, most often with a posterior sagittal anorectoplasty (PSARP operation) and can vary based on patient-specific factors, although MRI-assisted rectal pull-through has been described.
Cloacal Exstrophy
Cloacal exstrophy, the most severe anomaly in the bladder exstrophy-epispadias spectrum, is a rare congenital malformation. It is caused by a failure of fusion of the
infraumbilical midline structures at the cloacal stage of embryonic development. Cloacal exstropy is also known as OEIS syndrome (omphalocele-exstrophy-imperforate anus-spinal defects syndrome) because the four features are typically found together, which include omphalocele, exstrophy of the bladder and rectum, imperforate anus, and spinal defects. In affected children, many inner abdominal structures are exposed. In addition, diastatic symphysis pubis, spinal dysraphism, scoliosis, kyphosis, club feet and renal ectopia are also frequently present.
infraumbilical midline structures at the cloacal stage of embryonic development. Cloacal exstropy is also known as OEIS syndrome (omphalocele-exstrophy-imperforate anus-spinal defects syndrome) because the four features are typically found together, which include omphalocele, exstrophy of the bladder and rectum, imperforate anus, and spinal defects. In affected children, many inner abdominal structures are exposed. In addition, diastatic symphysis pubis, spinal dysraphism, scoliosis, kyphosis, club feet and renal ectopia are also frequently present.
 FIGURE 16.32 Neonate girl with an imperforate anus. Abdominal radiograph shows multiple dilated and gas-filled loops bowel to the level of rectum. The sacrum is normal. |
Currently, the diagnosis of cloacal exstrophy can be made by prenatal ultrasound or MRI, which can show the anterior opening of both the bladder and intestine and sometimes posterior opening of the spine. The diagnosis can be later confirmed on physical examination upon birth.
The current management choice of cloacal exstrophy is surgical repair that focuses on separation of the urinary and intestinal tracts, joining of the two hemibladders, colonic pull-through or colostomy, orchiectomy in genetic males, and closure of the abdominal wall defect.
Infectious Disorders
Esophagus
Infection of the esophagus, or esophagitis, occurs most often in immunocompromised children who have either congenital or acquired immunodeficiency. Currently, endoscopy (esophagogastroduodenoscopy [EGD], often with biopsy, has largely replaced esophagography as the preferred method for diagnosing infectious esophagitis. If possible, double-contrast technique should be performed to improve mucosal detail.
Viral Esophagitis
Cytomegalovirus
Cytomegalovirus (CMV) is a very common opportunistic pathogen in immunocompromised children that affects the esophagus and colon when there is GI tract involvement. Children with CMV esophagitis may present with odynophagia.52 Although EGD is the preferred diagnostic modality, esophagography can demonstrate large (sometimes >1 cm), shallow mid- or distal esophageal ulcers that are characteristic of CMV infection. Treatment is directed towards restoring immune competence and administering antiviral medications.52
Human Immunodeficiency Virus
Human immunodeficiency virus (HIV)-related esophageal ulcers are common and can often be attributed to CMV, herpes simplex virus (HSV), Candida albicans, and mycobacteria infections.53 Esophageal ulcers are termed HIV-related or idiopathic giant ulcers when the ulcer cannot be attributed to another cause. Affected patients usually present with odynophagia, dysphagia, and retrosternal chest pain, occasionally accompanied by hematemesis. Although HIV-related ulcers are difficult to differentiate from CMV-related ulcers by imaging, idiopathic lesions are typically larger and have overhanging edges in comparison to “punched-out” ulcers caused by CMV. These lesions usually occur in the mid- and distal esophagus. Medical management of HIV-related ulcers can include corticosteroids along with thalidomide in certain cases.54
Herpes Simplex Virus
HSV esophagitis also mostly occurs in immunocompromised children.55 Clinical symptoms include odynophagia and retrosternal pain. Esophagography usually reveals numerous tiny superficial ulcers, although ulcers can also appear punctate or linear. Severe HSV esophagitis can mimic Candida esophagitis. Treatment includes antiviral medication, such as acyclovir.56
Fungal Esophagitis
Candida albicans is the most common cause of infectious esophagitis. Oropharyngeal candidiasis and immunosuppression are common risk factors. Affected children usually present with odynophagia and dysphagia, and EGD often reveals raised white plaques with hyperemia and ulcerations in some cases. At esophagography, severe infection is characterized by a “shaggy” appearance of the esophagus, resulting from confluent longitudinally oriented plaques, pseudomembranes, and ulcers (Fig. 16.34). Treatment includes antifungal medications, such as nystatin and fluconazole. Other medications, such as amphotericin, are reserved for resistant cases in immunocompromised patients with fungemia.57
Stomach
Gastritis
Gastritis is a nonspecific term that referring to inflammation of the stomach wall (Fig. 16.35). In children, such changes can result from infection (e.g., Helicobacter pylori), chemical ingestion, eosinophilic disease, hypertrophic gastropathy, severe stress, or as the result of chronic conditions, such as Crohn disease and chronic granulomatous disease.
Peptic Ulcer Disease
Peptic ulcers are the result of a breach in the mucosal protective mechanisms of the stomach or duodenum that leads to ulceration. These ulcers may be a primary (secondary to H. pylori infection) or secondary (e.g., the result of stress, chemical ingestion, or gastrin-secreting tumor) in children.58 Peptic ulcers can affect the stomach or duodenum. Typical symptoms of peptic ulcers in young children include difficulty feeding and vomiting, whereas older children may present with GI tract bleeding or peritonitis due to viscus perforation.58 EGD is currently used to diagnose peptic ulcer disease.
Upper GI series can show large gastric and duodenal ulcers, although this examination lacks sensitivity for small ulcers especially if using single-contrast technique. Large ulcers may sometimes be apparent on CT, appearing as ulceration of the gastric or duodenal wall, adjacent viscus wall thickening, and perigastric or periduodenal inflammation (Figs. 16.36 and 16.37). Perforated ulcers may present with evidence of peritonitis on physical examination as well as pneumoperitoneum on CT (Fig. 16.37).59,60
Treatment of infectious peptic ulcer disease involves the eradication of H. pylori with antimicrobial agents and decreasing gastric acid production with proton pump inhibitors or histamine blockers.58
Small Intestine
Viral Infection
Infectious gastroenteritis due to viral pathogens is common. In the United States, viruses that cause gastroenteritis in the pediatric population are typically rotavirus, norovirus, and adenovirus.61,62 Viral gastroenteritis typically presents with vomiting followed by watery diarrhea, and in otherwise healthy children is self-limited and managed conservatively.63 Imaging studies generally are not necessary, because infectious diarrhea in children is usually a clinical diagnosis. However, in certain children where
symptoms are ambiguous and mimic other medical conditions, imaging may be performed to exclude other diagnoses, such as appendicitis.
symptoms are ambiguous and mimic other medical conditions, imaging may be performed to exclude other diagnoses, such as appendicitis.
Radiographic findings that may be indicative of viral gastroenteritis include nonobstructive bowel loop dilatation, absence of fecal material in the colon, multiple air-fluid levels, and, rarely, pneumatosis intestinalis64 (Fig. 16.38). Ultrasound and CT may show nonspecific small bowel wall thickening, distended fluid-filled bowel loops,65,66 and intraabdominal lymphadenopathy.62
Supportive care including oral rehydration is current management choice in children with viral gastroenteritis.
Bacterial Infection
Enteritis due to bacterial pathogens accounts for ˜2% to 10% of cases of infectious diarrhea in developed countries. The most common pathogens in the United States responsible for bacterial enteritis include Shigella, Salmonella, Escherichia coli, and Campylobacter.66 Common bacterial diarrhea-causing organisms in the developing world include Yersinia enterocolitica and Vibrio species. Symptoms of bacterial enteritis are similar to those of viral gastroenteritis, although bloody diarrhea, high fever, and chills are more commonly associated with bacterial pathogens like E. coli.66
Radiographic findings of bacterial enteritis most often involve the distal small bowel.65,67 Ultrasound may reveal nonspecific bowel wall thickening, hyperemia, and reactive free fluid. At CT, the small bowel typically shows bowel wall thickening, luminal narrowing, and perienteric inflammatory fat stranding (Fig. 16.39).
Treatment includes antibiotic therapy as well as fluid and electrolyte management.
Mycobacterial Infection
Abdominal mycobacterial infection is uncommon in the pediatric population, especially in otherwise healthy children. It can be caused by the Mycobacterium bovis (because of ingestion of unpasteurized infected milk products) or Mycobacterium tuberculosis (because of ingestion of infected sputum) organisms. Peritoneal tuberculosis (TB) is an uncommon extrapulmonary manifestation of M. tuberculosis infection that results from lymphohematogenous dissemination from a primary, usually pulmonary lesion. Abdominal TB involvement may present with abdominal distention secondary to ascites, pain, fever, and weight loss.68
Intestinal involvement by TB can have different imaging appearances, including ulcerative, hypertrophic, and mixed phenotypes. Among them, the ulcerative form is most common and is characterized by superficial ulcers. The hypertrophic form is characterized by bowel wall thickening and mural fibrosis. Ultrasound can reveal bowel wall thickening, mesenteric thickening, and lymphadenopathy. Affected lymph nodes may have hypoechoic central areas indicative of caseating necrosis.69 SBFT can be used to demonstrate small bowel and proximal colonic involvement, including associated strictures (Fig. 16.40). SBFT findings of intestinal TB infection include shortening of the ascending colon,
deformation of the cecum, and ileal thickening with a distorted ileocecal junction.70,71 Areas of intestinal narrowing and dilatation may also be observed.70,71 At CT, common findings of intestinal TB infection include distal small bowel wall thickening and right lower quadrant mesenteric lymph node abnormalities, which include enlargement, calcification, or central low attenuation (Fig. 16.40). Overall, imaging features of intestinal TB share substantial overlap with Crohn disease.
deformation of the cecum, and ileal thickening with a distorted ileocecal junction.70,71 Areas of intestinal narrowing and dilatation may also be observed.70,71 At CT, common findings of intestinal TB infection include distal small bowel wall thickening and right lower quadrant mesenteric lymph node abnormalities, which include enlargement, calcification, or central low attenuation (Fig. 16.40). Overall, imaging features of intestinal TB share substantial overlap with Crohn disease.
Treatment of intestinal TB is directed towards eradication of the infection.
Parasitic Infection
Two most commonly encountered small bowel parasites in the developed world include the protozoa Giardia lamblia and Cryptosporidium. Transmission generally occurs via the fecal-oral route through contaminated food and water.64 Parasitic worms, known as helminths, including nematodes (roundworms, such as ascarids, hookworms, and pinworms) and flatworms (such as schistosomes and tapeworms), are common intestinal pathogens in developing countries. These worms are estimated to infect up to one-third of the impoverished world population—most commonly children and adolescents72.
Small bowel fluoroscopic studies in children with parasitic disease can show dilution of contrast material from fluid retention, mucosal fold thickening, and aberrant transit time, either rapid or delayed, based upon the course of the disease.73,74,75 In addition, particular parasites are associated with specific imaging findings. For example, Giardia causes thickened duodenal and jejunal mucosal folds, rapid transit time, and dilution of contrast material.76 In Ascaris lumbricoides infection, contrast material may outline the parasitic organisms (Fig. 16.41). Live worms can also ingest contrast material, allowing for visualization of their intestinal tracts as well.76 A. lumbricoides worms also can be seen on ultrasound with the findings dependent on worm orientation, surrounding tissues, part of the worm imaged, and if the worm is alive or dead. Using low frequency transducers, the worm is seen as two parallel echogenic lines separated by anechoic area that represents the fluid-filled alimentary tract of the worm, and when using a high-frequency transducer, worms are seen as four parallel lines with three intervening anechoic spaces.77 In certain instances, the worms can be seen ingesting fluid during real-time imaging.77
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree