and John P. Deveikis2
(1)
Department of Surgery, Division of Neurosurgery, and Departments of Radiology and Neurology, University of Alabama, Birmingham, AL, USA
(2)
Bayfront Medical Center, St. Petersburg, FL, USA
Abstract
This chapters covers fundamental aspects of neurointerventional procedures, including preprocedural preparation, vascular access, antithrombotic management, post-procedural care, and complication management and avoidance. The Appendix discusses the neurointerventional suite.
This chapters covers fundamental aspects of neurointerventional procedures, including preprocedural preparation, vascular access, antithrombotic management, post-procedural care, and complication management and avoidance. The Appendix discusses the neurointerventional suite.
4.1 A Series of Unfortunate Events
One overriding principle should be kept in mind by every neurointerventionalist: Adverse outcomes always occur because of a series of minor deviations from standard practice, and rarely from only a single event.1,2 This simple truism has been noted in a wide range of human endeavors, from mountaineering3 to computer networks4 to aviation and space flight. A series of errors must occur in the correct order, and at just the right time, for a catas. For instance, a patient will never die only of an intracerebral hematoma after a neurointerventional procedure. That patient will have died because of: A) a failure to handle the exchange-length microwire properly, followed by B) failure to visualize the tip the of wire closely enough on fluoroscopy during the exchange, followed by C) a failure to recognize the immediate blood pressure and heart rate changes due to a wire perforation, followed by D) a failure to recognize a small area of contrast extravasation from a distal middle cerebral artery branch on the follow-up angiogram, followed by E) a failure to reverse the anticoagulation in a timely manner, and so on. Bad outcomes always result from a cascade of failures, never a single failure. Conversely, disaster may be avoided by recognizing components of this cascade early on, and avoiding making those decisions that compound the problem.
4.1.1 Tips for Preventing and Stopping a Cascade of Errors
1.
Always make sure that the planned procedure is truly indicated, and that the benefits outweigh the risks.
Example: Is coiling of a tiny unruptured asymptomatic aneurysm prudent? For a young patient with a previous history of subarachnoid haemorrhage, it may be; for an elderly patient with no other risk factors for haemorrhage, it may not be.
2.
Planning and preparation are mandatory.
Example: Airline pilots must submit a written checklist before each flight. If neurointerventionalists were required to do the same, then they might be safer.
3.
Always have a Plan B. And sometimes a Plan C.
Example: If Plan A is to coil an aneurysm that is also clippable, have a reasonable threshold to change to Plan B (surgery) if significant obstacles to coiling begin to appear.
4.
To avoid making mistakes, one should constantly assume one is going to make one.3 Approach every procedure with a humble mindset and the knowledge that whatever can go wrong, may well go wrong.
Example: During coiling of a ruptured basilar tip aneurysm, a routine guide catheter angiogram was done with injection of contrast into the RHV. Due to some excessive redundancy in the microcather, and because the RHV was not cinched down tightly (two small errors), the microcatheter tip was launched with the contrast injection through the dome of the aneurysm, and in spite of rapid coiling of the aneurysm and placement of a ventriculostomy, the patient died.
5.
Have a low threshold to STOP! when things begin to go wrong, particularly for non-emergent procedures. Regroup and recover from the first thing that went wrong.
Example: A sizable groin haematoma develops soon after the femoral artery puncture in a high-risk carotid stenting case. Solution: Stop, deal with the haematoma, and reattempt the stenting procedure another time.
6.
Encourage and accept input from everybody during procedures, including nurses, technologists, anesthesiologists, residents, fellows, and when appropriate, device company representatives.
Example: A visitor during a coiling case, observing from the control room, was the first to notice a clot forming at the tip of the guide catheter, which helped the operators during the case tremendously.
4.2 Preprocedure Preparation
General preparations for most neurointerventional procedures:
1.
Routine pre-procedure work-up:
a.
History and physical
b.
Neurological exam
c.
Imaging
d.
Blood work (CBC, Cr, PT, PTT)
e.
EKG
f.
Anesthesia evaluation, if needed
2.
Informed consent
3.
One or two peripheral IVs
4.
Foley catheter
a.
In the patient’s private room or pre-op area for awake patients
b.
In the angio suite, after induction of anesthesia, for asleep patients
5.
NPO after midnight or 6 h prior to the procedure except for medications.
6.
Make sure that all devices that may be needed are available in the angio suite prior to the procedure.
7.
Premedication:
a.
Dual antiplatelet therapy. Necessary for any stenting case, and an option for other interventions such as intracranial aneurysm coiling and liquid embolic procedures. See below (Antiplatelet therapy) for dosing.
b.
Protection against nephrotoxicity for patients with renal insufficiency (creatinine ≥1.5 mg/dL):
c.
Protection against anaphylaxis for patients with history of contrast allergy:
i.
Prednisone 50 mg PO (or hydrocortisone 200 mg IV) 13, 7, and 1 h prior to contrast injection.
ii.
Diphenhydramine (Benadryl®) 50 mg IV, IM or PO 1 h prior to contrast injection.
1.
Steroids should be given at least 6 h prior to the procedure; administration less than 3 h prior to the procedure does not reduce the risk of an adverse reaction.7
8.
Make sure that all devices that may be needed are available in the angio suite prior to the procedure.
4.3 Awake or Asleep?
Some operators prefer to use general anesthesia for most neurointerventional cases whereas others prefer to do them with the patient awake. Each approach has advantages and disadvantages. General anesthesia eliminates procedural discomfort, which can be substantial during some procedures, such as liquid embolic embolization and intracranial angioplasty. It also helps patients endure lengthy procedures, keeps them still for precise intracranial maneuvering, and simplifies the procedure for the operators somewhat, eliminating the need for constant coaching and neurological assessment of an awake patient. General anesthesia makes it possible to pause respirations intermittently, to obtain highly precise angiograms and roadmaps. However, general anesthesia makes it difficult to detect neurological changes in the patient, although electroencephalography and monitoring of somatosensory and/or motor evoked potentials can help remedy this. On the other hand, physiological monitoring during general anesthesia can cause crowding of the angio suite during procedures, and the reliability of monitoring is less than certain.
Doing neurointerventional procedures awake eliminates the risks associated with general anesthesia, permits constant monitoring of the patient’s neurological status, and reduces procedure time and room turnover time. Occasional patients simply cannot tolerate general anesthesia, most often because of cardiac or pulmonary disease. Shepherding an awake patient through a complex intracranial intervention, however, takes patience and skill on the part of the operator, and the judicious use of sedation and analgesia. Whatever the institutional practice is at any given center, it behooves the operator – and particularly those in training – to do cases awake occasionally to maintain the skills and comfort level necessary to do awake cases.
4.3.1 Awake
1.
Obtain IV access and a Foley catheter placement before the patient is brought to the angio suite.
2.
An abbreviated neurological exam is rehearsed with the patient prior to draping (e.g., patient is asked to say “Methodist Episcopal,” show their teeth and gums, wiggle their toes, and squeeze a rubber duckie (Fig. 4.1) with the hand contralateral to the side being treated).

Fig. 4.1
Rubber duckie. Other squeaky toys may be substituted if a duck is not available.
3.
Throughout the case, the patient is reminded to stay completely still. The patient’s head can be lightly taped to the head holder with a piece of plastic tape across the forehead, to remind him or her to stay still. Place a piece of non-adherent Telfa™ dressing (Kendall/Covidien, Mansfield, MA) between the tape and the forehead to ensure that the skin is not injured.
4.
Warn the patient prior to contrast injections or potentially uncomfortable catheter movements so there are no surprises.
5.
Check on the patient with gentle questions and reminders constantly throughout the procedure.
6.
Keep sedation and analgesia to a minimum to facilitate the patient’s full cooperation.
Options for sedation:
i.
Midazolam (Versed®) 1–2 mg IV for sedation; lasts approximately 2 h
ii.
Fentanyl (Sublimaze®) 25–50 μg IV for analgesia; lasts 20–30 min
4.3.2 Asleep
1.
Patient is placed under general anesthesia on the angiography table.
a.
Endotracheal anesthesia is most commonly used. Laryngeal mask anesthesia is also feasible, although the patient cannot be chemically paralyzed, and will move with respiration.
b.
If arterial blood pressure monitoring is needed during induction (e.g., when intubating patients with a ruptured aneurysm), then several options exist:
i.
Radial arterial line (SAH patients usually arrive in the angio suite with an A-line)
ii.
Femoral artery sheath. The sheath may be placed prior to induction and used to transduce blood pressure. A femoral artery sheath is less uncomfortable than a radial A-line.
2.
Strict attention to blood pressure is important during induction.
3.
If neurophysiological monitoring is used, baseline evoked potentials are obtained prior to the intervention. Depending on the anatomic location of the lesion, electroencephalography or monitoring of somatosensory, motor, visual or auditory evoked potentials may be useful. The authors of this handbook routinely use monitoring for procedures involving the spinal cord.
4.
The anesthesiologist is asked to report any abrupt changes in blood pressure or heart rate during the case, which can indicate intracranial haemorrhage.
5.
Following anesthesia, the patient’s neurological status is assessed.
4.4 Contrast Agents
1.
For most cases: Iohexol (Omnipaque®, GE Healthcare, Princeton, NJ) 240 mg I/mL. Patients with normal renal function can tolerate as much as 400–800 mL of Omnipaque®, 300 mg I/mL without adverse effects.8
2.
For patients with renal insufficiency: Iodixanol (Visipaque™, GE Healthcare, Princeton, NJ) 270 mg I/mL.
3.
See Chap. 2 for more detail about contrast agents, renal insufficiency, and iodinated contrast agent anaphylaxis.
4.5 Vascular Access
All neurointerventional procedures consist of 1) an access phase and 2) an intervention phase. The access phase usually consists of placement of a guide catheter in the carotid or vertebral artery via the femoral artery.
1.
The patient is placed on the angiography table.
2.
Dorsalis paedis and posterior tibialis pulses are assessed and marked.
3.
The right and left groins are clipped, prepped, and draped.
b.
Both groins are always prepped in case femoral artery access cannot be obtained on one side, or if a second sheath needs to be inserted.
c.
For sensitive patients, EMLA® (AstraZeneca, Wilmington, DE), a topical anesthetic cream, is applied over the expected puncture site 30 min prior to the procedure, and an occlusive dressing is placed.
4.
The PA C-arm is brought into position to enable fluoroscopy of the femoral artery, if needed for puncture of the vessel.
6.
A sheath is placed in the femoral artery.
a.
The size of the sheath depends on the intervention. For most intracranial cases, a 6F is suitable. A 7F sheath has the advantage of being large enough to accommodate a 6F guide catheter and still permit A-line transduction through the sheath.
b.
Sheaths are available in various lengths, most commonly 10 or 25 cm. The 25-cm version has the advantage that it bypasses any tortuosity in the iliac arteries. Having the distal end of the sheath in the aorta prevents any danger of injuring the iliac artery during catheter introduction through the sheath.
c.
Use a 0.038 J-tip wires (“Safety wires”) for sheath placement.
7.
If the use of a femoral artery closure device is planned, do a femoral artery angiogram at the beginning of the case right after insertion of the sheath, because the C-arm is positioned over the groin.
8.
If needed, do a diagnostic angiogram prior to the intervention.
Prior to the intervention, do intracranial PA and lateral angiograms, to serve as a baseline for later comparison, to check for the possibility of thromboembolism or haemorrhage in the intracranial circulation during or after the procedure.
9.
Obtain angiographic images of the access vessel (carotid or vertebral artery). Biplane imaging is best.
10.
Guide catheter selection. The authors prefer to use one of four guide catheters, depending on the situation:
a.
6F 0.053 in. Neuron™ Intracranial Access System (Penumbra, Inc., San Leandro, CA).
i.
Distal end: 5F OD, 3.9F ID. Comes in straight and angled shapes.
ii.
Advantages: Extremely soft and flexible; able to be positioned within the very distal intracranial ICA or vertebral artery.
iii.
Disadvantages: Less stable than other catheters, very slippery. Can be pushed out of the access vessel if the catheter is not in a distal-enough position. Only the distal tip is radio-opaque; the radiolucent shaft can be difficult to see on fluoroscopy. Narrow lumen makes for limited-quality angiograms with a microcatheter in position.
iv.
Technique and tips:
1.
Usually must be exchanged into position.
2.
Two lengths are available: 105 cm (for most patients) and 115 cm (for patients >6 ft in height).
3.
Two distal flexible zone lengths are available: 6 cm (for most cases) and 12 cm (for cases in which a very tortuous ICA or vertebral artery must be traversed, e.g., a cervical ICA with a 360° loop).
4.
A standard hydrophilic wire is used for initial positioning of the Neuron™.
5.
Coaxial microcatheter technique for final positioning of the Neuron™ 0.053 catheter:
Advance a microcatheter over a microwire through the Neuron™ into the target vessel distal to the desired final position of the guide catheter. Then advance the Neuron™ over the microcatheter to its final position. A more substantial microcatheter such as a Renegade® (Stryker Neurovascular, Fremont, CA) or Prowler® Plus (Codman Neurovascular, Raynham, MA) with a substantial 0.016 in. guidewire can provide good support to facilitate distal placement of the Neuron™.
6.
The more distal the tip, the more stable the Neuron will be; e.g., position the tip in the horizontal segment of the petrous ICA or the V4 segment of the vertebral artery for maximum stability. Optimal positioning is distal to at least two 90° turns in the vessel to provide sufficient support for the coaxial placement of a microcatheter.
7.
Guide catheter angiograms may be of marginal quality when a microcatheter is inside the guide catheter, because of the relatively narrow lumen. Injection of 100% contrast in a 3 mL syringe, rather than a 10 mL syringe, will produce better angiograms.
8.
The Neuron™ 053 will accept most microcatheters, but it may be difficult to inject contrast around 18-system microcatheters like the Renegade® (Stryker Neurovascular, Fremont, CA) or Prowler® Plus (Codman Neurovascular, Raynham, MA).
9.
Warning: When the Neuron™ is in its final intracranial position, use caution when flushing or injecting contrast. Use smaller volumes and lower pressures since the pressure is transmitted directly to the intracranial vessels. This can be particularly dangerous if there is an aneurysm nearby the catheter tip. Avoid using a power–injector while a microcatheter is in the Neuron™
b.
6F 0.70 in. Neuron™ Intracranial Access System (Penumbra, Inc., San Leandro, CA).
i.
Distal end: 6F OD, 0.70 in. (∼5.4F) ID. Comes in straight and angled shapes.
ii.
Advantages: Large lumen, able to accommodate two microcatheters (e.g., useful for balloon-remodeling). Permits good angiograms with a microcatheter in position.
iii.
Disadvantage: Relatively stiff, less navigable than the smaller Neuron. Straight tip means it usually has to be exchanged into position.
iv.
Technique and tips:
1.
Usually must be exchanged into position.
2.
Two distal flexible zone lengths are available: 6 and 8 cm.
3.
Can be advanced into a distal position by placing a Distal Access Catheter (DAC®, Concentric Medical, Mountain View, CA), inside the Neuron, which eliminates the large step-off between the tip of the Neuron and the guide wire.
c.
Guider Softip™ XF guide catheter (Stryker Neurovascular, Fremont, CA):
i.
Advantages: Soft, atraumatic tip. Minimizes risk of vasospasm and dissection in narrow, tortuous vessels. Angled tip allows it to be navigated into position primarily, without an exchange.
ii.
Disadvantages: Relatively flimsy, prone to fall into the arch when the vasculature is tortuous.
d.
Envoy® (Codman Neurovascular, Raynham, MA):
i.
Advantages: Relatively rigid, provides a good platform in tortuous vessels, large internal lumen. Nice for working in the external carotid artery. Angled tip allows it to be navigated primarily.
ii.
Disadvantages: Stiff, sharp-edged tip.
11.
Alternative guide catheters:
a.
6 Fr 90 cm Cook Shuttle® (Cook, Inc., Bloomington, IN):
i.
Very large, stable platform.
ii.
Technique:
1.
Requires either an 8F sheath, or an exchange for a smaller sheath (e.g., for carotid stent cases). If a smaller sheath (e.g. 5F sheath) is placed first, a diagnostic catheter is then placed in the access artery. The diagnostic catheter is then exchanged over an exchange-length hydrophilic wire for the Cook Shuttle®, with the obturator still in place. When the Cook is ∼2 cm proximal to the desired final position, the obturator is removed and several mL of blood are allowed to spill backwards out of the sheath, to remove any bubbles or clot. Note: the obturator is not radio–opaque.
2.
Tip: if the Cook winds up in the aortic arch, access to the great vessels can be obtained by advancing a hydrophilic wire and a 125 cm 5F Vitek catheter within the Cook. The Vitek has a shape similar to a Simmons 2 catheter, and can be used to navigate the Cook back into the carotid or subclavian arteries.
b.
Merci® Balloon Guide catheter (Concentric Medical, Mountain View, CA)
i.
Capable of temporarily occluding flow in the carotid or vertebral artery during thrombectomy procedures using the Merci® Retrieval system or the Solitaire™ stent.
1.
Available in 7, 8 and 9F sizes; the 7F is recommended for a small vertebral artery and the 9F is for a large carotid artery.
2.
It is packaged with an obturator for smooth vessel access. The proximal end of the guide catheter includes a straight hub for device insertion and an angled hub for distal balloon inflation.
3.
The compliant silicone balloon at the distal end of the guide catheter inflates to a 10 mm diameter with the maximum inflation volume of 0.8 mL is used. A 3 mL syringe, prepared with 50% contrast in saline, is used for balloon inflation.
4.
Caution: This guide catheter is flimsy and is prone to fall into the aortic arch when significant counter-force is generated by the microcatheter.
c.
Neuron™ MAX 088 Large Lumen Catheter (Penumbra, Inc., San Leandro, CA)
i.
Large catheter, available either as a long sheath (for use like the Cook Shuttle) or a guide catheter. Four cm distal tip is more flexible than the Cook.
ii.
Available in 80 and 90 cm lengths.
iii.
Technique:
1.
Obtain access to the target vessel (carotid or vertebral artery) with a diagnostic catheter first, then exchange the MAX 088 into position over an exchange-length hydrophilic wire.
2.
The MAX 088 comes with an inner dilator. A larger Neuron Select catheter can also be used within it.
d.
ReFlex™ (Reverse Medical Corporation, Irvine, CA)
i.
2 sizes are available, 0.58 in. and 0.72 in.
ii.
Very similar to the Neuron™ Intracranial Access System.
e.
Berenstein Large Lumen Balloon Guide Catheter (Boston Scientific, Natick, MA)
i.
Advantages: 11.5 mm diameter balloon allows for proximal flow control, to prevent distal migration of embolic agent in high flow states.
ii.
Disadvantages: Relatively small lumen (despite it’s name). Short length (80 cm) limits the use to short patients and a very proximal catheter position.
f.
Pinnacle® Destination® (Terumo Medical, Somerset, NJ)
i.
Advantages: Designed as a long sheath to also act as a guide catheter. Inner dilator provides a smooth transition to guidewire as it is advanced. Relatively rigid to provides a very stable platform. Large lumen. An inner guide catheter can be placed to provide added stability (“Tower of Power”).
ii.
Disadvantages: Rigid sheath should not be placed too distally or in small vessels to prevent vessel injury. Somewhat less rigid and less stable than other systems.
g.
6F Northstar® Lumax® Flex Catheter (Cook, Inc., Bloomington, IN):
i.
Device contour consists of a smooth, tapered transition between the guidewire, inner dilator, and catheter, which minimizes trauma to vessel walls. The dilator also allows introduction without the use of a groin sheath. Relatively rigid, providing a stable platform.
ii.
Disadvantages:
1.
Relatively stiff.
2.
Extremely lubricious (may cause the catheter to slide out of vessels).
12.
Guide catheter size:
a.
The guide catheter should be 90 cm long (and not longer) for use with the Wingspan stent system.
b.
6F for most cases.
c.
5F if the vessel caliber is small and collateral circulation is limited:
i.
e.g., for use in a small vertebral artery when the contralateral vessel is hypoplastic.
ii.
Disadvantage: Angiograms with a microcatheter or balloon in place are more difficult to obtain because of limited space within the guide catheter.
13.
Straight or angled?
a.
Straight guide catheter is useful in relatively straight vessels, or in situations where the guide catheter is gently navigated through a convoluted vessel:
i.
Usually requires exchanging (see below).
ii.
Preferred for the vertebral artery.
b.
Angled guide catheter is useful when the final position of the catheter tip is in a vessel curve:
c.
Angled catheters are easier to navigate through the aortic arch than straight catheters.
14.
Guide catheter placement technique:
a.
The guide catheter is typically placed in the ICA or vertebral artery only after the heparinization is therapeutic (usually 5 min or more after the IV loading dose is given).
b.
Exchange method:
i.
Usually necessary for the Neuron™ guide catheters and other straight guide catheters, because the absence of an angle at the tip make it difficult to navigate the catheter primarily. It is also useful in patients with tortuous anatomy, atherosclerosis, or fibromuscular dysplasia. This technique minimizes risk of injury to the carotid or vertebral artery, particularly at the vessel origin.
ii.
Guide a 5F diagnostic catheter into the CCA or vertebral artery over an exchange-length (300 cm) 0.035 in. or 0.038 in. hydrophilic wire.
iii.
The tip of the hydrophilic wire is advanced into a distal branch of the ECA or the distal extracranial vertebral artery (usually the first 90° turn of the vessel at C2) using road mapping technique.
iv.
The diagnostic catheter is then gently removed while the tip of the hydrophilic wire is continuously visualized on fluoroscopy.
v.
The hydrophilic wire is wiped down with a dripping-wet Telfa™ (Kendall/Covidien, Mansfield, MA) sponge.
1.
Avoid wiping hydrophilic wires with dry cotton sponges. It leaves numerous thrombogenic cotton fibers on the wire.
vi.
The guide catheter is advanced over the wire while continuously visualizing the tip of the wire.
c.
Direct navigation method:
i.
Possible in patients with nontortuous, nonatherosclerotic vessels.
ii.
Navigate an angled guide catheter gently into the carotid or vertebral artery over a 0.035 or 0.038 in. hydrophilic wire.
15.
Guide catheter position
a.
Carotid artery
i.
Using a roadmap, advance the guide catheter over a hydrophilic wire into the ICA as distally as possible. A “high position” of the guide catheter will maximize the stability of the guide and improves control over the microcatheter and microwire. In a non-tortuous, healthy carotid system, the authors of this handbook prefer to position the tip of the guide catheter in the vertical segment of the petrous ICA. In a cervical ICA with a significant curve in the vessel, the guide can be adequately positioned immediately proximal to the curve. Moderate curves in the vessel can be straightened out by guiding a relatively stiff hydrophilic wire (e.g., an 0.038 in. wire) through the affected segment, followed by the catheter, but this may compromise flow in the vessel due to kinking or spasm.
b.
Vertebral artery
i.
Using a roadmap, position the guide catheter in the distal extracranial vertebral artery, usually at the first curve (at C2).
c.
Once the guide catheter is in position, do a gentle injection of contrast through the guide catheter under fluoroscopy, to examine the configuration of the vessel around the tip and to check for the presence of vasospasm or vessel dissection around the tip. If catheter tip-induced vasospasm is present and flow-limiting, withdrawal of the catheter tip by several millimeters is often sufficient to restore flow.
16.
Keep the tip of the guide catheter in view on one or both biplane fluoroscopic views for the duration of the case. Correct displacement of the guide catheter tip, and, if the catheter appears to unstable, consider replacement with a more stable guide catheter system.
17.
Guide catheter care and maintenance
a.
Guide catheter irrigation
i.
Continuous irrigation of the guide with heparinized saline (10,000 units of heparin per liter of saline) is important.
ii.
Meticulous attention to the RHV and the guide catheter is necessary throughout the procedure is necessary to identify thrombus or bubbles, should they appear.
b.
Tips to minimize or treat guide catheter-induced vasospasm:
i.
Withdraw the catheter into a lower segment of the vessel when significant catheter-induced vasospasm appears.
ii.
Keep the catheter tip away from kinks and bends in the vessel if possible.
1.
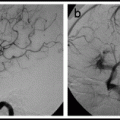
A curvaceous carotid or proximal vertebral artery can sometimes be straightened out by tilting the patient’s head toward the opposite shoulder (Fig. 4.2).
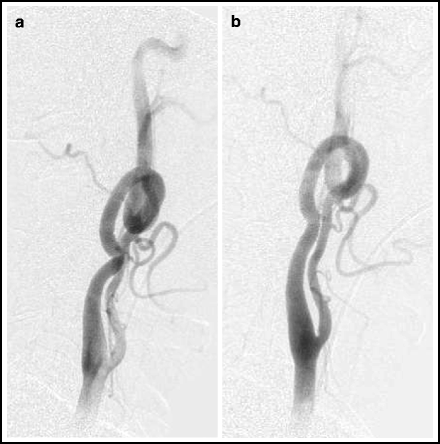

Fig. 4.2
Head tilt technique. Lateral angiogram of left carotid system in a neutral position (left) and with the head tilted toward the opposite shoulder (right).
iii.
Selective injection of IA nitroglycerin (30 μg per injection)
1.
This can also help distinguish vasospasm from vessel dissection, if a dissection is suspected
iv.
Use Visipaque™ (GE Healthcare, Princeton, NJ) contrast instead of Omnipaque; according to the manufacturer, this contrast material is less spasmogenic than Omnipaque®.
v.
Use a soft-tipped guide catheter.
vi.
Use a guide catheter with an inner obturator (e.g., Northstar® Lumax® Flex Catheter.Cook, Inc., Bloomington, IN).
4.5.1 Tips for Difficult Access Cases
1.
Femoral artery is not accessible.
a.
Use an alternative route (see below)
2.
Aortic arch or great vessels are tortuous
a.
Use the ECA to anchor the wire.
b.
During the initial placement of the diagnostic catheter in the CCA, use a 0.035-in. hydrophilic wire to advance the diagnostic catheter into a branch of the ECA.
c.
Then remove the wire and replace it with a stiffer exchange-length wire, such as a 0.038-in. hydrophilic wire or an Amplatz stiff wire.
d.
Then exchange the diagnostic catheter for the 90-cm sheath.
e.
This technique works well in the left CCA using a Simmons 2 catheter as the diagnostic catheter.
3.
“Tower of power” technique to add stability to the 90-cm sheath:
a.
Advance a 6F guide catheter, e.g., 6F Envoy (Codman Neurovascular, Raynham, MA), inside of an 6F 90-cm sheath.
b.
Larger diameter 90-cm sheath (e.g., 7 or 8F) will add stability.
c.
Use an intermediate catheter (e.g. DAC®, Stryker Neurovascular, Fremont, CA) inside a guide catheter inside a 90 cm sheath to create the ultimate “Tower of power”.
4.
Buddy wire technique.
a.
Use a larger diameter 90-cm sheath (e.g., 8F sheath) and a 0.014 or 0.018-in. wire anchored into the subclavian artery or a branch of the ECA.
4.5.2 Alternative Access Routes
If the femoral artery cannot be accessed (e.g., because of high grade iliac or femoral artery stenosis or occlusion, innominate or subclavian artery tortuosity, patients unable to tolerate laying flat, morbid obesity, or aortic disease) several other options exist. The arm approach is nicely suited for access to the ipsilateral vertebral artery. For access to the great vessels from the arm, use a 5F Simmons 2 catheter to access the target vessel (see Fig. 2.7), then exchange a guide catheter into position over an exchange-length 0.035 in. or 0.038 in. hydrophilic wire. See Chap. 2 for further details about techniques.
1.
Brachial artery.10
a.
Advantages:
i.
Large enough for 6 and 7F sheaths.
ii.
Hand ischemia is usually not a threat.
iii.
Large enough to use a closure device.
1.
Off-label use of the Perclose® Pro-glide™ (Abbott Vascular, Abbott Park, IL, Inc.) in the brachial artery is feasible.
b.
Disadvantages:
i.
Median nerve injury is a potential complication, because of the proximity of the nerve to the brachial artery.
2.
Radial artery.11
a.
Advantages:
i.
Easier to get haemostasis after the sheath is removed, compared to the brachial artery approach.
ii.
Less potential for nerve injury.
3.
Axillary artery.
4.6 Antithrombotic Therapy During Neurointerventional Procedures
The overall risk of thromboembolic complications during neurointerventional procedures is significant. Aneurysm coiling, for example, carries a 2–8% risk of symptomatic thromboembolic complications.18–21 Strategies for antithrombotic medication management vary widely. Anticoagulation with heparin, to some degree or another, is standard for most procedures. Dual antiplatelet therapy is standard for all stenting procedures. Some operators also advocate routine antiplatelet therapy (e.g., aspirin) for many neurointerventional procedures, such as aneurysm coiling.
4.6.1 Anticoagulation
1.
Heparin for flushes and irrigation.
a.
Use heparinized saline 10,000 units of heparin per liter of saline, (except in paediatric patients < 6 years of age, then use 1,000 units of heparin per liter of saline)
2.
Systemic anticoagulation:
a.
Loading dose of IV heparin (5,000 units or 70 units/kg) prior to placement of the guide catheter in the access vessel. Additional heparin is given during the procedure, if necessary, 1,000 units IV every hour.
b.
Monitoring of activated clotting time (ACT): 5 min after giving IV heparin, draw a 5 mL specimen of blood for an activated clotting time (ACT) from the sheath. The target ACT range is 250–300 s. If the first ACT is not within the target range, additional heparin may be given and the ACT re-sent. The guide catheter is placed in the ICA or vertebral artery only after the heparinization is therapeutic (usually 5 min or more after the IV loading dose is given, or after the ACT has been found to be in the target range). Additional doses of heparin (1,000 units/h) may be given for longer cases.
c.
ACT monitoring is a neurointerventional tradition with little scientific foundation, and some operators do not routinely check the ACT.
3.
Alternatives to heparin
a.
Argatroban (Novastan®, Abbott, North Chicago, IL) is an antithrombotic suitable for use in patients with heparin induced thrombocytopaenia.24,25 Coronary interventional doses of 350 μg/kg bolus over 3–5 min have been used for neurointerventional procedures, and adequacy of antithrombotic effect is monitored by ACT values around 250–300 s. A continuous drip of 10–25 μg/kg/ min can be used for longer procedures, or, alternatively, 150 μg/kg boluses at hourly intervals if the ACT falls below 250. The saline infusions through the catheter or sheath lumen must obviously not contain any heparin. Argatroban has no specific antidote, and the only course of action to employ in the case of active bleeding is to stop the infusion and wait for the effect to wear off. Therefore this agent must be used with caution.
b.
Bivalirudin (Angiomax™, The Medicines Company, Cambridge, MA) is a synthetic direct thrombin inhibitor that is popular in interventional cardiology and has been used in neuro-endovascular procedures in select cases.26 It can also be used in patients who cannot tolerate heparin, as in cases of heparin-induced thrombocytopaenia.27 However, like argatroban, there is no rapid reversal agent for bivalirudin, and its routine use in patients for intracranial procedures is not recommended.
c.
Other designer antithrombotics that can be used in patients with heparin contra-indications include lepirudin and danaparoid.28
In some hospitals these unusual antithrombotic medications may have restrictions on their use and may require a Haematology consult in order to obtain the drug from the pharmacy.
4.6.2 Antiplatelet Therapy
Thrombosis in the arterial system is of the platelet-rich “white clot” variety; therefore, antiplatelet therapy during neurointerventional procedures makes sense. Dual antiplatelet therapy helps prevent stent thrombosis in patient undergoing endovascular stenting procedures,29 and treatment with aspirin and clopidogrel is commonly used for any patient undergoing a neurointerventional stenting procedure. Recent evidence suggests that oral antiplatelet therapy may reduce thromboembolic complications in patients undergoing coiling of intracranial aneurysms,30–33 although it may carry an increased risk of haemorrhage.34
1.
Aspirin
Standard loading and maintenance dose: 325 mg PO QD. Available as a suppository.
2.
3.
Dual antiplatelet therapy
a.
Aspirin 325-mg PO QD for ≥3 days prior to the procedure and
c.
Aspirin 325-mg PO QD for ≥3 days prior to the procedure and
d.
Ticlopidine (Ticlid® Lilly, Indianapolis, IN) for ≥3 days prior to the procedure.40
1.
Adverse reactions include rash, gastrointestinal side effects, neutropaenia (2.4%), thrombocytopaenia, aplastic anemia, and thrombotic thrombocytopaenic purpura.41
2.
Neutropaenia occurs in 2.4% of patients and may appear within a few days.
i.
Monitoring for neutropaenia: CBC with absolute neutrophil count and peripheral smear should be done prior to initiation of therapy and every 2 weeks through the third month of therapy
e.
Alternatively, a loading dose of Aspirin 325 mg PO and clopidogrel 600 mg PO can be given the day before or at least 5 h before the procedure.
4.
Glycoprotein IIB/IIIA inhibitors.
a.
Treatment with IV or IA GP IIB/IIIA inhibitors are useful in two situations:
b.
Abciximab (ReoPro® Merck & Co., Whitehouse Station, NJ)
i.
Dose: 0.25 mg/kg IV rapid bolus followed by 125 μg kg/min infusion (to a maximum of 10 mg/min) for 12 h.
ii.
Caution: Partial dosing of Abciximab should be avoided, unless point of care testing confirms adequate receptor blockade. 45 The authors recommend the use of a full loading dose followed by IV infusion for 12 h, unless the threat of haemorrhagic complications is prohibitive. The authors of this handbook have personally witnessed paradoxical drug-induced platelet activation effect with lower levels of platelet inhibition with abciximab, and a corresponding increase in thrombotic complications.
iii.
The effect of Abciximab can be reversed, if needed, with platelet transfusion. The drug is a monoclonal antibody, with high affinity to the GP IIB/IIIA receptor on platelets.
c.
Eptifibitide (Integrilin® Lilly, Indianapolis, IN)
i.
ii.
Eptifibitide cannot be reversed with platelet transfusions. It is a small-molecule drug with low affinity for the GP IIB/IIIA receptor.
4.6.3 Clopidogrel Resistance and Platelet Function Testing
Clopidogrel is a thienopyridine antiplatelet agent. It acts by irreversibly inhibiting the platelet surface P2Y12 adenosine diphosphate (ADP) receptor. Blockade of the P2Y12 receptor prevents aggregation of platelets and cross-linking by fibrin by preventing activation of the GP IIb/IIIa receptor.47 Clopidogrel is a prodrug; after absorption by the duodenum, it is metabolized by the cytochrome P450 system in the liver to the active metabolite, R-130964. Some 5–10% of ingested clopidogrel is converted to the active metabolite.48 Polymorphisms of at least two genes involved with clopidogrel have been found to influence either the bioavailability of the drug or it’s effect on platelets. Polymorphisms in the ABCB1 gene, which codes for a glycoprotein involved with the passage of clopidogrel across the duodenal wall, have been found to influence the bioavailability of clopidogrel.49,50 The CYP2C19 gene codes for a hepatic esterase responsible for converting clopidogrel to the active metabolite; >33 different alleles of the CYP2C19 gene have been identified. Each allele is defined by variations in the DNA sequence, which may result in functional differences in the CYP2C19 enzyme.51 The CYP2C19*1 allele is common in people of European origin and the CYP2C19*2 allele is present in 30%, 15% and 17% of Asian, Caucasian, and black patients, respectively.52 Carriers of CYP2C19*2 have reduced effectiveness of clopidogrel53,54 and have an elevated risk of cardiovascular events and coronary stent thrombosis.55 Because of these findings the FDA issued a safety warning in May, 2009 that clopidogrel has reduced effectiveness in patients who are poor metabolizers of the drug.52,56 Aside from genetic tendencies, other causes of poor responsiveness to clopidogrel include noncompliance, drug interactions,57 inadequate absorption,58 body mass index,37,59 and increased platelet activity related to an acute thrombotic event.60 Platelet activation is a significant threat in neurointervention, contributing to overall rates of acute thromboembolic events up to 8%.18,21,61,62
Several techniques are available for so-called point-of-care detection of poor responsiveness to antiplatelet therapy.63 Among the most commonly used are the VerifyNow™ Rapid Platelet Function Assay (Accumetrics, San Diego, CA), Innovance® Platelet Function Analyzer (Siemens Healthcare Diagnostics, Inc., Deerfield, IL) and the Multiplate® Multiple electrode aggregometry device (Dynabyte Medical, Munich).63 Point-of-care testing is meant to identify patients who are at higher risk of thromboembolic complications during percutaneous interventional procedures. Point-of-care testing has become de rigueur in some cardiac cath labs and neuroangio suites, but the vast majority of published experience with point-of-care testing has been from cardiology.64,65
Several studies of point-of-care platelet function testing in neurointerventional procedures have been published. In a series of 50 patients undergoing cerebrovascular stenting procedures, 28% were classified as clopidogrel nonresponders, and there was a significant correlation between clopidogrel nonresponse (as assessed by the Multiplate® device) and procedural adverse events.66 In another study of 76 patients undergoing cerebrovascular stenting procedures, clopidogrel resistance (as assessed by the VerifyNow™ assay) was reported in 51.9%.67 A study of 186 aneurysm coiling patients found that diminished clopidogrel responsiveness (by VerifyNow™) correlated with thromboembolic events.62 Another study of 216 neurointerventional patients found that inadequate platelet inhibition (by VerifyNow™) was found in 13% of patients on aspirin and 66% of patients on clopidogrel.68 Yet another study of 106 neurointerventional patients found that 42.9% were poor responders to clopidogrel (by VerifyNow™), and that all cases of intraprocedural thrombosis occurred in the poor-response group.37
4.6.4 Should Routine Platelet Function Testing Be Done Prior to Neurointerventional Procedures?
Point-of-care platelet function testing is controversial. In the medical management of ischemic stroke, recent editorials have argued for and against platelet function testing.69–71 A number of publications have concluded that routine platelet function testing is not justified.51,63 No standards, guidelines, or randomized trial data exist for the use of platelet function testing for neurointervential procedures, yet some operators use it routinely while others do not. This practice remains an option until more definitive data on the question is available.
1.
Get Clinical Tree app for offline access

Arguments in favour of routine point–of–care platelet function testing:
b.
Acute thrombosis due to platelet activation is a significant source of morbidity.
c.




Point-of-care testing is relatively easy and feasible.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree