and John P. Deveikis2
(1)
Department of Surgery, Division of Neurosurgery, and Departments of Radiology and Neurology, University of Alabama, Birmingham, AL, USA
(2)
Bayfront Medical Center, St. Petersburg, FL, USA
Abstract
Provocative testing attempts to predict what, if any, clinical deficit would result from the occlusion of some vessel or resection of the territory supplied by that vessel. Provocative testing may be mechanical, in which a vessel is temporarily occluded, usually using a balloon, or pharmacologically, in which an agent is injected to temporarily anesthetize and inactivate a neuroanatomical territory in the brain, spinal cord, or a nerve. When the provocative test is being done, the patient is examined to check for new neurological deficits that may result from either the lack of blood flow to a vascular territory in the case of balloon test occlusion, or an anesthetic infusion into the neural tissue supplied by the vessel being tested pharmacologically. These procedures may be done preoperatively or as part of a therapeutic endovascular procedure to ensure the safety of occluding a vessel by open surgical or endovascular methods. This chapter focuses on arterial procedures.
Provocative testing attempts to predict what, if any, clinical deficit would result from the occlusion of some vessel or resection of the territory supplied by that vessel. Provocative testing may be mechanical, in which a vessel is temporarily occluded, usually using a balloon, or pharmacologically, in which an agent is injected to temporarily anesthetize and inactivate a neuroanatomical territory in the brain, spinal cord, or a nerve. When the provocative test is being done, the patient is examined to check for new neurological deficits that may result from either the lack of blood flow to a vascular territory in the case of balloon test occlusion, or an anesthetic infusion into the neural tissue supplied by the vessel being tested pharmacologically. These procedures may be done preoperatively or as part of a therapeutic endovascular procedure to ensure the safety of occluding a vessel by open surgical or endovascular methods. This chapter focuses on arterial procedures. See Chap. 12 for venous provocative testing.
6.1 Balloon Test Occlusion
6.1.1 Background
Temporary occlusion of a vessel has been shown to be a safe, predictable way to estimate the effect of permanent vascular occlusion. Test occlusion is done to predict whether occlusion of the vessel will have negative haemodynamic consequences, which can result in ischaemic injury to neural tissue and result in a permanent functional deficit. Temporarily occluding a vessel to predict the functional effects was first reported by Rudolph Matas, a New Orleans surgeon, in the early twentieth century, and therefore, the test occlusion procedure is sometimes referred to as the Matas test.1, 2 The use of an endovascular balloon allows for reversible occlusion of the vessel in a predictable fashion. Balloon test occlusion is generally performed prior to endovascular or surgical occlusion of a major cerebral artery in the management of aneurysms, tumours and other neurosurgical problems.
There are two conditions that must be met to ensure the reliability of the test occlusion results:
1.
The vessel being occluded must be at the proper site and level to simulate the anticipated permanent occlusion. It is important to test-occlude beyond any potential collateral vessels that may still provide flow to the brain during the test, yet may be lost after more distal permanent occlusion. In the carotid circulation, more than half the population has angiographically apparent branches of the proximal intracranial carotid that can be a pathway for collateral flow to the brain, during a test occlusion in the cervical carotid.3 The ophthalmic artery is a significant collateral pathway in many patients and some patients who pass a test occlusion with a balloon proximal to the ophthalmic, may fail when the balloon is placed at the level of the ophthalmic, occluding the collateral flow via that vessel.4 A simple rule of thumb is to perform a test occlusion of a vessel with balloon inflation at the same level as the anticipated permanent occlusion.
2.
The test should reliably predict neurological consequences of the vascular occlusion. Temporary occlusion of a vessel could sufficiently lower the blood flow to an eloquent neuroanatomical region, so that a demonstrable neurological deficit occurs. The situation is simple if the test result is abnormal, and the patient exhibits a neurological deficit during the test: it is very likely that the patient would suffer some haemodynamic ischaemic injury due to permanent occlusion of that vessel. When a neurological change occurs during a test occlusion, it may not always be true that a permanent deficit would occur with permanent occlusion of the artery, thanks to the potential for collateral enlargement (arteriogenesis) after occlusion. However, it is never wise to ignore a test occlusion that produces a deficit. Somewhat more problematic is the situation in which no deficit occurs during the test occlusion. Does this imply that the patient will never have an ischaemic problem from permanent occlusion of the vessel, or is there a potential for false negative test occlusions? Neurological examination during arterial test occlusion is usually combined with additional manoeuvers, when possible, to corroborate the clinical findings. These additional manoeuvers include cerebral blood flow imaging, acetazolamide administration, and pharmacological lowering of blood pressure.
Carotid artery test occlusion is done frequently, and experience with the procedure has shown the predictive power of the test. A systematic review of 254 patients in five studies in which an internal carotid was therapeutically sacrificed without a test occlusion found an average stroke rate of 26%, and mortality of 12%.5 These results are in contrast to a study of 262 patients in eight studies in which the internal carotid was occluded after performing a test occlusion with an average stroke rate of 13% and mortality of 3%. This difference in stroke and death rate reached statistical significance. The significant morbidity associated with carotid artery occlusion, even with prior test occlusion, indicates that test occlusion is an imperfect predictor with a significant false negative rate. Adjunctive evaluation techniques (the additional manoeuvers mentioned above) were added to the neurological assessment to reduce the chances of a false negative test occlusion. The rationale of the additional these tests is that occlusion of the carotid or other vessels may produce a drop in blood flow that puts the patient at risk for stroke, yet not enough of a drop to produce detectable neurological dysfunction during a trial occlusion for a reasonable period of time. These adjunctive tests look for subtle signs of neurological dysfunction or look for the effects of the vessel occlusion on blood flow to the target territory.
6.1.2 Adjunctive Tests of Neurological Function
1.
Hypotensive challenge.6–10 Lowering the blood pressure magnifies the haemodynamic effect of vascular occlusion, making it more likely that a neurological deficit will occur in the case of limited collateral flow. When the carotid artery is occluded and no deficit occurs in a normotensive patient, the blood pressure is pharmacologically lowered to a target pressure (e.g. 66% of mean baseline pressure6), or until the patient develops a focal neurological deficit or becomes too nauseated and uncomfortable to allow adequate clinical assessment. Agents that can be used for lowering blood pressure should be fast-acting and quickly reversible, such as nitroprusside or esmolol.
(a)
Advantages: Cheap and easy to perform. Does not require moving the patient from the angiography suite.
(b)
Disadvantages: Headaches and nausea are common, for the patient (and the physician). A small series.10 had 15% false negative rate, which is no better than just a clinical test occlusion.
2.
Neuropsychological testing. 9, 11, 12 In addition to simple neurological testing during temporary vessel occlusion, a battery of standardized neuropsychological tests are given to test higher cortical functions.
(a)
Advantages: Cheap and easy to perform. Can be performed in the angiography suite. Standardized tests of higher cortical function can detect subtle signs of neurological dysfunction, even if the patient does not have an apparent motor or sensory deficit.12
(b)
Disadvantages: Requires skilled personnel to administer testing in an accurate and consistent manner. Most centers have limited experience with this test. Accuracy is unproven.
3.
Electroencephalography (EEG). 13, 14 Continuous EEG monitoring is done throughout the procedure. Slowing or other deviations from baseline conditions can be secondary signs of developing ischaemia.
(a)
Advantages: Does not require moving the patient with the balloon in place. Can still be done with patients under light general anesthesia. Monitored results can be recorded and examined carefully at a later time, to look for changes corresponding to events during the procedure.
(b)
Disadvantages: Adds cost and complexity to the procedure. Requires pre-placement of EEG leads prior to starting the procedure. Requires skilled personnel to monitor the readings. Careful neurological testing will almost always reveal a deficit when EEG changes are present, making the use of this modality redundant when the patient is awake and can be tested neurologically.
4.
Somatosensory evoked potentials (SSEP) 15, 16 EEG electrodes are attached and electrical stimulation of a peripheral nerve (usually the median nerve) contralateral to the hemisphere being tested, is performed. Cortical responses are recorded and the timing and amplitude of the response indicates cortical function. Testing is done prior to and following balloon inflation.
(a)
Advantages: Does not require moving the patient with the balloon in place. Can still be done with patients under light general anesthesia. Monitoring results can be recorded and examined carefully at a later time to look for changes corresponding to events during the procedure.
(b)
Disadvantages: Adds cost and complexity to the procedure. Requires pre-placement of EEG leads and nerve stimulation leads prior to starting the procedure. Stimulation of the nerve can be uncomfortable and distracting to the patient. Results may be difficult to interpret in the setting of underlying spinal or peripheral nerve disease. Requires skilled personnel to monitor the readings. The value of this test is unclear, compared to standard neurological testing.
5.
Cerebral Oximetry. 17, 18 Commercially available cerebral oximeter, such as INVOS® (Somanetics, Troy, MI) can be applied to the forehead and allows measurement of frontal lobe oxygenation.
(a)
Advantages: Does not require movement of the patient with the balloon in place. Results seem to correlate with neurological deficits and SPECT imaging.17
(b)
Disadvantages: Gives only a limited evaluation of frontal lobe oxygenation. Results can be affected by underlying brain pathology. Sensitivity and specificity are unproven.
6.1.3 Adjunctive Tests of Blood Flow
1.
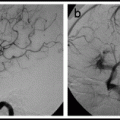
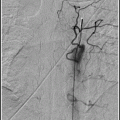
Angiography. 19, 20 Cerebral angiography before and during balloon test occlusion allows qualitative, semiquantitative assessment of brain blood flow and potential collateral circulation to the occluded vascular territory. A posterior communicating artery diameter <1 mm is a risk factor for subsequent stroke with carotid occlusion.21 Similarly, the absence of a functional anterior communicating artery is a risk factor for haemodynamic stroke after carotid occlusion.22 Semi-quantitative assessment consists of looking for synchronous filling of cortical veins with angiography of the contralateral carotid or vertebral during trial occlusion of a carotid, and measuring the difference between hemispheres in the time it takes to achieve venous filling.19, 20 This provides a rough approximation of differences in mean transit time between the hemispheres.
(a)
Advantages: Easily done. Does not require moving the patient with the balloon in place. Can limit the time the balloon needs to be inflated. Can be done in patients who are under general anesthesia.
(b)
Disadvantages: Requires use of a second catheter to obtain arteriograms of contralateral carotid and vertebral arteries while the balloon is inflated. This then requires bilateral groin punctures or the use of a GuardWire® (Medtronic Vascular, Santa Rosa, CA) balloon wire and diagnostic catheter placed via the same femoral sheath (as discussed below). Results are somewhat subjective. Published data on the accuracy of this, compared to more direct measurements of blood flow, are lacking.
2)
Back-pressure (“stump-pressure”) measurement. 23,24 Blood pressure is measured through the end-hole of the catheter distal to the site of balloon occlusion. The absolute value of the back-pressure or better yet, a ratio of minimum mean back-pressure to mean systemic blood pressure can be recorded. A ratio of 60% or greater is indicates good collateral flow and predicts tolerance to occlusion.25
(a)
Advantages: Quick and easy. Does not require moving the patient with the balloon in place.
(b)
Disadvantages: Requires the use of a double lumen balloon catheter, with a central lumen for a guidewire or pressure measurements, and another lumen for inflating and deflating the balloon. Stump pressure fluctuates over time as the balloon is inflated and may not absolutely correlate with Xenon-CT data.23 Back-pressure readings can be affected if the balloon catheter is in a curve and the end-hole of the catheter kinks or presses against the vessel wall.
3.
Transcranial Doppler (TCD) 26–28 Sonographic evaluation of the middle cerebral artery is obtained before and after balloon inflation. Mean blood flow velocity and pulsatility index that do not decrease more than 30% are highly predictive of tolerance to carotid occlusion.27
(a)
Advantages: Does not require moving patient with the balloon in place. Can be done in patients under general anesthesia.
(b)
Disadvantages: Adds cost and complexity to the procedure. Visualization of the intracranial vessels can be time consuming and can distract from examination of the patient. Test results may be difficult to interpret in the setting of underlying vascular disease. Requires skilled personnel to perform the study, and results can be operator-dependent. Unproven value compared to standard neurological testing.
4.
133 Xenon imaging 29 Radioactive Xenon is administered while the carotid is occluded and the patient’s brain is imaged with a detector, and blood flow data calculated.
(a)
Advantages: Can provide quantitative blood flow data.
(b)
Disadvantages: Gives only whole-brain images, so only gross side-to-side differences are visible. Use of the radioactive xenon is cumbersome.
5.
Xenon CT 5, 30 Dynamic CT imaging is done as the patient inhales non-radioactive xenon gas. Scans are obtained prior to balloon inflation to determine baseline flow, and the study is repeated during balloon occlusion to determine the effect of occlusion on blood flow. Can also be done with acetazolamide injection during balloon inflation to evaluate for the presence of vascular reserve.
(a)
Advantages: Provides accurate blood flow data reliably. The hardware used for the xenon delivery is compatible with most commercially available CT scanners. Can do repeated scans to allow scans with and without balloon inflation, and also after acetazolamide.
(b)
Disadvantages: May require moving a patient into the CT scanner with the balloon in place. The scan may be done without transferring the patient if the angiography and balloon placement is done on the CT scanner table using a portable C-arm.31) Xenon gas is not FDA approved, and therefore currently requires an Investigational Drug Exemption (IDE) and all the associated paperwork. The hardware for delivering the gas and software for the CT computations require experienced personnel to obtain good studies. Xenon gas can produce euphoria, agitation, and/or nausea in patients, making it difficult to avoid patient motion, which greatly affects accuracy. Produces images of only a limited area of the brain.
6.
CT perfusion 32 Dynamic CT imaging with a bolus of intravenous contrast, and post-processing can provide blood flow, blood volume and mean-transit time. Scans are obtained without balloon inflation to determine baseline flow, and the study is repeated with balloon inflation to determine the effect of occlusion on blood flow. CT perfusion can also be done with acetazolamide injection during balloon inflation to evaluate cerebrovascular reserve. See the Primer on Imaging in Stroke in Chap.9 for more detail.
(a)
Advantages: Readily available on most CT scanners. Quick and easy. Uses standard iodinated contrast used for any intravenous contrast-enhanced scan. Blood flow data has been validated by Xenon-CT.33 Can do repeated scans to allow scans with and without balloon inflation and also after acetazolamide.
(b)
Disadvantages: May require moving a patient into the CT scanner with the balloon in place. Requires large-bore intravenous access. Multiple perfusion studies can add to the amount of iodinated contrast given. Produces images of only a limited section of the brain.
7.
Positron emission tomography (PET) 34 Short acting, radioactive tracers such as.15O H2O are administered and PET scanning done. Postprocessing allows blood flow calculation. Scans are obtained without balloon inflation to determine baseline flow, and the study is repeated with balloon inflation to determine the effect of occlusion on blood flow. Can also be done with acetazolamide injection during balloon inflation, to evaluate for the presence of vascular reserve.
(a)
Advantages: Can give accurate quantitative blood flow data. Can image the entire brain, allowing for visualization of secondary signs of impaired cerebral blood flow, such as crossed cerebellar diaschisis.35 Crossed cerebellar diaschisis is a reflexive drop in blood flow to the contralateral cerebellar hemisphere, when a substantial drop in blood flow to a cerebral hemisphere occurs. Can do repeated scans to allow scans with and without balloon inflation and also after acetazolamide.
(b)
Disadvantages: Requires moving the patient to the PET scanner with the balloon in place. PET scanners are not universally available. Requires immediate access to a cyclotron for making the radiotracer, such as.15O H2O, which has a very, very short half-life. Cyclotrons are even more scarce than are PET scanners. To allow for quantitative blood flow measurements, requires an arterial access such as a larger diameter femoral sheath or a separate radial arterial line.
8.
Single-photon emission computed tomography (SPECT) 36–39 99mTechnicium-HMPAO is injected intravenously within 5 min after the balloon is inflated, and the radioactive tracer deposits in the brain in quantities proportional to the regional blood flow. After the test occlusion is completed, SPECT scanning shows activity from the tracer, and asymmetry is detected qualitatively by visual inspection of the scan and by measuring the number of radioactive counts in each region of interest.
(a)
Advantages: Quick and easy. The imaging can be done after the procedure is completed, so there is no need to transport the patient with a balloon in the vessel. The entire brain can be imaged, allowing for visualization of secondary signs of impaired cerebral blood flow, such as crossed cerebellar diaschisis.40, 41
(b)
Disadvantages: Does not allow for accurate quantitative measurement of cerebral blood flow. Reliance on asymmetry without absolute values of cerebral blood flow can result in significant false positive and false negative results.42 Scans obtained with the balloon inflated and deflated cannot be obtained immediately one after the other.
9.
Magnetic resonance (MR) perfusion 43,44 Diffusion-weighted scans, perfusion imaging with a bolus of intravenous gadolinium contrast, and post-contrast T1 weighted and FLAIR imaging are performed prior to and following the balloon inflation. The diffusion and post-contrast scans are observed for signs of ischaemia with the balloon inflated. Calculation of cerebral blood volume, mean transit time and blood flow can be done on a computer workstation using the MR perfusion data. See the Primer on Imaging in Stroke in Chap. 9 for more detail.
(a)
Advantages: Does not add to the iodinated contrast given to the patient. Can image the entire brain.
(b)
Disadvantages: Requires MR compatible balloon catheters and patient monitoring leads. Unless one has a combined MR and angiographic interventional suite, requires transfer of patient while the balloon is in place. Quantitative blood flow data is of uncertain validity. Significance of any changes on diffusion imaging or postcontrast scans is uncertain.
10.
Computer simulation 45, 46 Proprietary software allows computer modeling of blood flow in the intracranial circulation using data from MR and digital subtraction angiography imaging.
(a)
Advantages: A small series showed computer flow modeling showing greater than 20% drop in flow in the M1 segment and A3 segment during carotid occlusion, accurately predicted the patients who developed clinical symptoms during test occlusion.46 May theoretically replace invasive balloon-test occlusion.
(b)
Disadvantages: Unproven efficacy.
The bottom line on adjunctive tests: Nothing is perfect. Use at least one or two adjunctive tests in addition to neurological assessment. In most cases the two adjunctive tests are angiography plus cerebral blood flow imaging.
6.1.4 Indications for Test Occlusion
To determine the potential safety of occluding an artery, prior to treatment for:
1.
Intracranial haemorrhage
2.
Aneurysm
3.
Arteriovenous malformation
4.
Arteriovenous fistula
5.
Tumours involving a vascular structure
6.1.5 Complications of Balloon Test Occlusion
Informed consent prior to the procedure should include an estimate of the risk of complications.
6.1.5.1 Neurological Complications
1.
Thromboembolic stroke; a series of 500 carotid test-occlusions reported 1.6% symptomatic neurological complications, of which two (0.4%) were permanent.30
2.
3.
Overly aggressive balloon inflation in intracranial vessels can rupture the artery.
4.
In the cavernous ICA, carotid cavernous fistula can result from overinflation of the balloon.
6.1.5.2 Nonneurological Complications
1.
Balloon inflation in the carotid bulb or basilar artery can produce a vasovagal reaction and bradycardia, hypotension, and rarely, cardiac arrest.
2.
Balloon inflation in the basilar artery can produce unconsciousness and apnea.
3.
Anaphylactic reactions to iodinated contrast or any of the medications used can occur as with any endovascular procedure.
4.
Similarly, groin hematomas, femoral or iliac dissections.6, puncture site infections or other access complications can occur.
5.
Use of hypotensive challenges may theoretically provoke cardiac ischaemia.
6.1.6 Balloon Test Occlusion: Technique
6.1.6.1 Preprocedure Preparation
1.
Informed consent.
2.
IV access.
3.
Foley catheter.
4.
Rehearse a brief neurological exam with the patient prior to the case.
5.
Place a rubber duckie (see Fig. 4.1) in the contralateral hand if the carotid territory is going to be tested.
6.
Sedation and analgesia should be minimized if neurological assessment during the test occlusion is planned.
6.1.6.2 Vascular Access Phase
See Chap. 4 for a general discussion of access techniques.
1.
A 6F sheath is placed in the femoral artery.
(a)
If a second catheter is needed for angiography of collateral vessels during the test occlusion, place a 5F sheath in the contralateral femoral artery.
2.
Guide catheter selection.
(a)
6F Guider (Stryker Neurovascular, Fremont, CA) or Envoy® (Codman Neurovascular, San Jose, CA) are stiff enough to provide support when positioned in the common carotid artery, and have an inner diameter to permit good angiograms when the balloon catheter is in position.
(b)
Sheaths (90 cm) (e.g. Shuttle® sheath, Cook Inc., Bloomington, IN) also work very well as alternative guiding catheters for test occlusions. This allows for extra stability if needed.
3.
Guide catheter access is obtained in the usual fashion.
4.
Antithrombotic medication. A loading dose of IV heparin is given (5,000 units or 70 units/kg is given after sheath placement and at least 5 min before the test occlusion.
5.
Pretreat with 0.3–0.5 mg atropine if planning on inflating a balloon in the carotid bulb or basilar artery and the baseline heart rate is low.
6.1.7 Balloons for Test Occlusion
There are four main categories of devices for test occlusion:
1.
Double lumen balloon catheter. This has a central lumen for the microwire and for pressure measurements, and another lumen for inflating and deflating the balloon. These devices are similar to most angioplasty balloons, but high-pressure, low-compliance angioplasty balloons are not recommended for test occlusions, as they can traumatize the vessel. Soft balloons such as those on standard occlusion balloons or even Swan-Ganz balloon catheters can be used. The advantage of these balloons is the ability to measure pressures through the distal lumen, and also these balloons are relatively inexpensive. The disadvantage of these balloons is that they do not manoeuver well, are somewhat traumatic to vessels and consequently should not be used in very small vessels or intracranial vessels, although The Ascent™ (Codman Neurovascular, San Jose, CA) is designed to be used in intracranial vessels.
2.
Over-the-wire microballoon. The prototypical balloon in this category is the Hyperform™ (ev3 Neurovascular, Irvine, CA) (see Fig. 11.1). The balloon catheter has single lumen and when the appropriately sized wire is advanced beyond the catheter tip, an O-ring type valve in the balloon catheter seals the tip of the catheter around the wire and allows inflation and deflation of the balloon. These balloons have the advantage that they are soft, atraumatic, and very manoeuverable to almost any destination. The downside is that they have single lumen to inflate the balloon and no way to measure backpressure when the vessel is occluded. These small balloons are advanced through a guide catheter placed in the proximal carotid or vertebral artery, depending on the vessel being tested. Measurement of pressure through the guide catheter will show dampening of the pressure waveform, if the balloon is inflated in the vessel a short distance beyond the guidecatheter tip. The microwire must be advanced through the balloon catheter for at least a short segment distal to the balloon. Thus, it requires a straight segment to place the distal wire and care should be taken to keep the wire tip out of small side-branches or acute bifurcations, to prevent perforations or dissections. The risk of injury caused by the microwire can be minimized by creating a tight J-shaped curve on the guidewire tip. One advantage of the microwire in these single lumen balloons is that, if necessary, the balloon can be rapidly deflated by withdrawing the wire. Other balloon types do not have this option for rapid deflation. These microballoons are the most common balloons currently used for cerebrovascular test occlusions.
3.
Inflatable balloon wire. This is typified by the GuardWire® (Medtronic Vascular, Santa Rosa, CA). This system works well especially for carotid test occlusions. This occlusion balloon is mounted on a 0.014-in hypotube (a small diameter wire with an inner lumen) and has a 0.028-in profile for the 2.5–5-cm balloon, or 0.036 in for the 3–6 mm balloon. The larger balloon can easily be advanced through 6F guide catheter. It is inflated with an inflation device, which can be removed from the wire, leaving the balloon inflated. This allows removal of the guide catheter and permits placement of a diagnostic catheter via the same femoral arterial sheath for performance of control angiography of potential collateral vessels, while the balloon occludes the target vessel. Another advantage of this balloon wire is that, it has such a low profile that it allows safe moving of the patient with the balloon in place, for cerebral blood flow imaging with CT perfusion. The disadvantages of the balloon wire is that the distal stump pressure cannot be measured and the wire tip extends for several centimeters distal to the balloon. The GuardWire® has other disadvantages, including the fact that it is stiffer than the microballoons, so, as a general rule, should not be used in intracranial vessels or other small vessels. The need for the inflation device also means that there is a bit of a learning curve to be able to use this device efficiently. The most annoying problem with this device is the length of time required for balloon deflation. The balloon should be inflated with a dilute contrast solution (e.g. 30% contrast in saline) to minimize viscosity and decrease the problems associated with inflating and deflating the balloon.
4.
Detachable coils. In extremely small, tortuous distal vessels, it may not be possible to safely advance even the smallest microballoons. However, these vessels may still be accessible using low-profile, ten-system microcatheters. With the microcatheter in the vessel to be tested, a detachable coil can be advanced into the vessel to temporarily occlude it. This method will only work in vessels <3 mm in diameter. Use a 2 or 3 mm diameter coil that is stretch resistant so that it can be easily removed. The coil should also be ultra-soft, to fill the lumen of the vessel without traumatizing the intima. Advance as few loops of coil as necessary to occlude flow. Obviously, the patient must be fully heparized, so that the thrombus does not form in the vessel, and occlusion times must be kept to a minimum. Given the possibilities of thrombus formation and the remote possibility of coil stretching or inadvertent detachment, this method should not be routinely used for test occlusion unless everything else fails.
Balloons must be sized to match the vessel being occluded. Measure the target vessel using a previous angiographic study, or obtain an angiogram as part of the procedure to get a measurement of the vessel.
1.
The ICA requires balloons at least 5 mm in diameter in most cases.
2.
The extracranial ICA can be occluded with Swan-Ganz double-lumen balloon (Edwards Lifesciences, Irvine, CA), 10 mm occlusion balloon catheter (Cook Medical, Bloomington, IN), 7 × 7 mm Hyperform™ microballoon (ev3 Neurovascular, Irvine, CA), or 6 mm GuardWire®(Medtronic Vascular, Santa Rosa, CA).
3.
The intracranial ICA is best occluded with a Hyperform™ microballoon (ev3 Neurovascular, Irvine, CA) or the Ascent™ (Codman Neurovascular, San Jose, MA).
4.
The vertebral arteries can usually be occluded with 5 mm diameter or larger balloons.
5.
The straight segment of the cervical vertebral can be occluded with a 10 mm occlusion balloon or a 7 × 7 mm microballoon listed above.
6.
Above the C2 segment of the vertebral artery, where the artery curves laterally, only flexible microballoons such as the Hyperform™ should be used.
7.
Intracranial vessels such as ICA and vertebral arteries may be as large as 4–5-mm in diameter. The supraclinoid ICA is usually 3.5 mm and basilar artery is usually 3.2 mm in diameter. More distal branches are generally <3 mm in diameter.
8.
Vessels >4 mm can be occluded with the 7 × 7 mm Hyperform™ microballoon, or 6 × 9 mm Ascent™ (Codman Neurovascular, Raynham, MA).
9.
Vessels ≤4 mm may be occluded with the 4 × 7 mm Hyperform™ (ev3, Irvine, CA), a 4 × 10 mm Hyperglide™ microballoon (ev3, Irvine, CA) or 4 × 10 mm Ascent™ (Codman Neurovascular, San Jose, CA).
6.1.8 Guidewires
Steerable hydrophilic wires such as 0.035 or 0.038-in. angled Glidewire® (Terumo Medical, Somerset, NJ) can be used to advance a diagnostic catheter or guiding catheter into the carotid or vertebral artery.
A 0.025 or 0.035-in. Glidewire® (Terumo Medical, Somerset, NJ) can be used for standard occlusion balloons.
The Transend™ 10 (Stryker Neurovascular, Fremont, CA), X-pedion™ 10 (ev3, Irvine, CA), or other 0.010-in. microwire is used to advance an over-the-wire microballoon catheter to the target vessel.
The GuardWire® (Medtronic Vascular, Santa Rosa, CA) balloon is integrated as part of a 0.014-in. wire, and can be used as a wire for relatively soft catheters.
6.1.9 Catheter and Balloon Manipulation
1.
Attach all the catheters to RHVs and attach a continuous infusion of saline containing 10,000 units heparin per liter.
2.
Through the femoral (or brachial) sheath, advance the guide catheter into the ICA or vertebral artery.
3.
Do angiograms to determine the best position for the balloon, and to size the artery for proper balloon selection.
4.
Avoid positioning the balloon in any area containing atherosclerotic plaque.
5.
Obtain a roadmap mask to allow balloon positioning and inflation under roadmap guidance.
6.
Warn patients that the catheter manipulation and balloon inflation may cause a feeling of pressure.
7.
Use extreme caution when advancing or inflating a balloon in intracranial vessels.
8.
Always keep track of where the tip of the guidewire is to avoid vascular perforation or dissection.
9.
When the balloon reaches the desired location, pull back on the catheter to remove any slack. This will prevent the balloon from advancing forward as it is inflated.
10.
Inflate the balloon only just enough to stop the flow. Do not overinflate.
11.
Measure the volume required to inflate the balloon and occlude the vessel. When doing adjunctive blood flow imaging, requiring patient transfer with the balloon in place, this allows for deflation and inflation of the balloon without fluoroscopic control.
6.1.10 Double-Lumen Balloon Catheter Technique
1.
Prepare the balloon by attaching a 10 mL syringe partially filled with contrast to the inflation port of the balloon, aspirate any air, and release suction to allow contrast to enter the inflation port.
2.
Attach a one-way stopcock to the inflation port, and inflate the balloon with 50:50 contrast:saline mixture, then deflate. Angle the balloon to allow aspiration of any residual air as the balloon is deflated.
3.
For those ambitious (or foolish) enough to use a Swan–Ganz (Edwards Lifesciences, Irvine, CA) for test occlusion, expect to struggle getting into the vessel of interest, as these balloons are not designed for arterial catheterization. A 0.010 in. microwire can be used to direct the catheter and to do partial inflations for flow direction, but it is not a pleasant experience in tortuous vessels.
4.
For all other balloons, such as the 10 mm occlusion balloon catheter (Cook Medical, Bloomington, IN), it is usually necessary to use an exchange wire, unless the patient is young or has straight cervical arteries that are easily accessible with a straight catheter.
5.
Using a diagnostic angiographic catheter of desired size and shape, such as a 5F Angled Glidecath® (Terumo Medical, Somerset, NJ), catheterize the target carotid or vertebral artery that is to be tested.
6.
Using a 300 cm, 0.035 in. diameter exchange wire, exchange the diagnostic catheter for the balloon catheter.
7.
Advance the balloon catheter so that it is just proximal to the site of intended occlusion, and inject the contrast through the central lumen to obtain a roadmap of the vessel.
8.
Advance the balloon to the target site.
9.
Prepare to measure pressures through the end-hole of the balloon catheter, either by attaching a pressure line to the stopcock (or manifold) attached to the central lumen of the balloon catheter, or by using a pressure-sensing guidewire.
10.
Measure a baseline pressure through the central lumen of the balloon catheter.
11.
Gently inflate the balloon just enough to occlude the vessel.
12.
Inject contrast through the end-hole of the balloon catheter; pooling of contrast in the artery will confirm complete occlusion.
13.
Measure the pressures through the central lumen of the balloon catheter again. When the vessel is occluded, there will be dampening of the pressure waveform. A drop in mean pressure by 50% after balloon inflation is suggestive of insufficient collateral flow to the distal territory.
14.
Examine the patient for any neurological deficits and pay particular attention to functions performed by areas of the central nervous system supplied by the vessel being occluded.
15.
At some point during the test occlusion, do a cerebral angiogram using an arterial catheter in a contralateral sheath, and check for collateral flow from other arterial pathways.
16.
If the patient tolerates the balloon inflation clinically and the back pressure in the balloon catheter does not drop <50% post-inflation, keep the vessel occluded for an extended period (∼30 min) to confirm tolerance to occlusion.
17.
Consider using a supplementary test to look for other signs of haemodynamic insufficiency, when the balloon is inflated (see below).
18.
Signs of test occlusion failure:
(a)
Neurological changes
(b)
Drop in back-pressure
(c)
Evidence of poor collateral flow by angiography
(d)
Adjunctive test evidence of poor collateral flow
19.
The procedure is complete when the patient fails the test occlusion, or if they pass for at least 30 min. The balloon should be deflated.
20.
Prior to removing the balloon, ensure that the patient’s symptoms have resolved. If not, a dissection or a thromboembolic complication may have occurred, and leaving the balloon catheter in place provides access for diagnostic angiography and corrective intervention.
21.
Remove the balloon catheter when all testing is complete.
6.1.11 Microballoon Catheter Technique
1.
Prepare the Hyperform™ or Hyperglide™ (ev3 Neurovascular, Irvine, CA) by thoroughly flushing the sterile holder housing the balloon to activate the hydrophilic coating.
2.
Attach a one-way stopcock, or Flo-switch (BD Medical, Franklin Lakes, NJ) to a rotating haemostatic valve, and attach this to the balloon catheter.
3.
Fill the balloon catheter with 50% contrast diluted with saline.
4.
Insert the X-pedion™ (ev3 Neurovascular, Irvine, CA) or other 0.010-in. wire through the rotating haemostatic valve and into the balloon catheter.
5.
Make a j-tip curve on the shapeable platinum tip of the wire, to limit the risk of the microwire traumatizing or perforating a vessel.
6.
Place the guide catheter in the carotid or vertebral artery.
7.
Make a roadmap.
8.
Advance the balloon to the target site.
9.
Gently inflate the balloon just enough to occlude the vessel.
10.
Confirm occlusion of the vessel by injecting the contrast through the guide catheter. There will be stasis of the contrast in the vessel proximal to the balloon.
11.
Examine the patient for neurological deficits and pay particular attention to functions performed by areas of the central nervous system supplied by the artery being occluded.
12.
At some point during the test occlusion, do a cerebral angiogram using an arterial catheter in a contralateral sheath. Check for collateral flow from other arterial pathways.
13.
If the patient tolerates the balloon inflation clinically, keep the vessel occluded for an extended period (∼30 min) to confirm tolerance to occlusion.
14.
Use adjunctive tests to assess for haemodynamic insufficiency when the balloon is inflated (see below).
15.
Get Clinical Tree app for offline access
Signs of test occlusion failure:
(a)
Neurological changes
(b)
Drop in back-pressure
(c)




Evidence of poor collateral flow by angiography
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree