and John P. Deveikis2
(1)
Department of Surgery, Division of Neurosurgery, and Departments of Radiology and Neurology, University of Alabama, Birmingham, AL, USA
(2)
Bayfront Medical Center, St. Petersburg, FL, USA
Abstract
Extracranial embolization procedures are therapeutic endovascular occlusions of vessels outside the cranial cavity. This chapter covers head and neck transarterial embolization procedures, percutaneous procedures, and spinal embolization.
Extracranial embolization procedures are therapeutic endovascular occlusions of vessels outside the cranial cavity. This chapter covers:
1.
Head and neck transarterial embolization procedures
2.
Percutaneous procedures
3.
Spinal embolization
8.1 Head and Neck Transarterial Embolization
8.1.1 Indications
1)
Bleeding
a)
Idiopathic epistaxis (exceedingly common)
b)
Post-traumatic (common)
c)
Post surgical (occasionally)
d)
Bleeding tumours (occasionally)
e)
Post-radiation changes (occasionally)
2)
Carotid blow-out syndrome (a particularly problematic combination of (c)–(e), above)
3)
Extracranial vascular tumours, pre-operative embolization (common) or palliative embolization (rare)
a)
Juvenile nasopharyngeal angiofibroma
b)
Paraganglioma (aka chemodectoma, glomus tumour)
c)
A wide variety of other primary and metastatic vascular tumours.
4)
Extracranial arteriovenous malformations (AVM) (uncommon)
a)
Superficial AVMs
b)
Intraosseous AVMs
c)
Diffuse AVMs
d)
Intraorbital AVMs
8.1.2 Relative Contraindications
1.
Feeding vessels feed eloquent structures (e.g., brain, eye, or spinal cord)
2.
Vascular anatomy that is difficult for endovascular access (e.g., exaggerated vessel tortuosity, vascular anomalies).
3.
Significant atherosclerotic disease or high-flow vasculopathy affecting the parent vessel (e.g., occlusion or stenosis of the access vessel).
4.
Life-threatening contrast allergy.
5.
Coagulation disorders or heparin hypersensitivity.
6.
Active bacterial infection (i.e., bacteremia at time of endovascular treatment).
8.2 Techniques and Devices
8.2.1 Evaluation
1)
History and physical.
2)
Neurological exam.
3)
Blood work (CBC, BUN, Creatinine, PT, PTT)
4)
Imaging
a)
CT or MRI of the lesion
b)
CTA or MRA
c)
If possible, a catheter angiogram.
d)
Imaging considerations
i)
Lesion location, potential territories at risk from the procedure, size and configuration.
ii)
Whether there is involvement of the skull or spine.
iii)
Flow patterns (e.g., high flow vs. low flow arteriovenous shunt).
iv)
Parent vessel anatomy.
v)
Angiographic architecture of lesion (e.g., nidal AVM vs. high-flow AVF vs. mixed lesion vs. tumour)
vi)
Presence of associated vascular lesion (e.g., aneurysms, multiple AVMs or AVFs).
vii)
Plan site of intended deposition of embolic material.
viii)
Access vessel anatomy.
ix)
Presence or absence of stenosis in the access vessel.
8.2.2 Treatment Strategy
Assess the patient and available imaging prior to the case, and preferably prior to the day of the procedure. Decisions about the overall treatment strategy and the role of embolization in that strategy should be made well ahead of time. Plans should include:
1)
Choice of access vessel
2)
Guide catheter selection
3)
Microcatheter and microwire selection
4)
Embolic agent to be used
5)
Target vessels to be treated
6)
Single procedure versus staged multiple procedures
7)
Preparation for methods to ensure preservation of neurological function (e.g., provocative testing, neuophysiological monitoring)
8.2.3 Preprocedure Preparation
1)
One or two peripheral IVs.
2)
NPO for 6 h, except for medications.
3)
Patients on insulin for hyperglycemia should get half their normal dose prior to the procedure.
4)
Place Foley catheter.
5)
Make sure that all devices that may be needed are available in the angio suite prior to the procedure.
6)
Many practitioners routinely pre-treat patients with dexamethasone, 2 mg PO/IV Q 6 h for 2–3 days before any tumour embolization procedure to attempt to reduce the risk of swelling.
7)
Patients with a recent hemorrhage:
a)
Arterial line and large-caliber venous access are established prior to the procedure.
b)
If the airway is threatened, the patient should be intubated and ventilated.
8.2.3.1 Awake or Asleep?
1)
As a rule, extracranial embolization procedures should be done awake.
2)
Exceptions: Children, patients with unstable airways (e.g., some epistaxis patients) and cases that are anticipated to be very long in duration.
8.2.4 Vascular Access Phase
See Chap. 4 for a general discussion of access techniques.
1)
A 6F sheath is placed in the femoral artery.
a)
A 7F sheath is necessary if arterial monitoring is going to be done with the sheath, or if a need for a proximal balloon catheter is anticipated. An even larger sheath may be needed if stent-grafting is going to be done.
2)
Systemic anticoagulation.
a)
Thromboembolic complications can occur during any vascular catheterization. The senior author of this handbook favours universal use of systemic heparin for all embolization procedures whereas the junior author almost never uses heparin.
i)
Who is right, and who is wrong? Systemic anticoagulation with IV heparin appears to carry relatively little risk in patients without active bleeding, and judicious use of heparin appears to be of relatively low-risk particularly since the drug can be rapidly reversed with protamine.
ii)
IV heparin dose: 5,000 units or 70 units/kg, followed by 1,000 units per additional hour.
3)
Guide catheter selection
a)
Large-bore, stiff guide catheters are best for external carotid artery positioning and embolization. The large ID permits the use of large microcatheters and allows for good-quality angiograms with injection of contrast into the guide catheter while the microcatheter is in position.
i)
Envoy® (Codman Neurovascular, Raynham, MA)
ii)
Guider Softip™ XF guide catheter (Stryker Neurovascular, Fremont, CA)
iii)
Cook Shuttle® (Cook Medical, Bloomington, IN)
iv)
4F Berenstein II (Cordis Endovascular, Miami Lakes, FL)
(1)
This guide catheter is particularly useful for external carotid territory embolizations because it can easily be placed in the distal external carotid or even superselectively in individual branches with little trauma to the vessels. It acts like a mini-Envoy®.
4)
Guide catheter positioning
a)
Carotid system.
i)
Using a roadmap, advance the guide catheter over a hydrophilic wire into the ECA and into a straight segment proximal to the origin of the branch feeding the lesion. A “high position” of the guide catheter maximizes the stability of the guide catheter and improves control over the microcatheter and microwire. When the target involves proximal branches of the ECA, like the ascending pharyngeal artery or the superior thyroid artery, it may be necessary to position the tip of the guide catheter in the distal segment of the common carotid. In a very curved ECA, the guide catheter can be should be positioned immediately proximal to the curve. Moderate curves in the vessel usually cannot be straightened out by guiding a relatively stiff catheter around them, and the guide catheter can cause spasm or even a dissection. Therefore, it is better to settle for a relatively proximal guide catheter position. If added stability is necessary, a relatively stiff 0.014 in. wire can be advanced through the guide catheter as a “buddy wire,” and if the catheter has a large enough lumen, a microcatheter can be advanced along-side it. The buddy wire will help keep the guide catheter in place.
b)
Subclavian artery.
i)
Using a roadmap, position the guide catheter adjacent to the target branch. The thyrocervical and costocervical trunks may sometimes be directly catheterized with a guide catheter, but more often, the guide catheter must be placed in the proximal subclavian artery.
ii)
A buddy wire is often needed during embolizations with the guide catheter in the proximal subclavian artery.
c)
Do a guide catheter angiogram once it is in position to assess the configuration of the vessel around the tip and to check for the presence of vasospasm or vessel dissection around the tip. If catheter tip-induced vasospasm is present and flow limiting, withdrawal of the catheter tip by several millimeters is often sufficient to restore flow.
d)
Attached an RHV with continuous saline infusion to the hub of the catheter.
8.2.5 Microcatheter Access Phase
Once a stable guide catheter position has been obtained, advance a microcatheter is advanced into a position from which embolic material can be delivered to the target.
1)
Working views
a)
Obtain high magnification PA and lateral working views and make sure that the guide catheter is visible on at least one view.
b)
Use biplane roadmaps during navigation.
2)
Microcatheter selection
a)
There are many microcatheters, and the optimal choice depends on how large or how distal the target vessel is, what embolic agent will be used, and the training and experience of the operator. See Chap. 7 for more detail about microcatheters.
b)
Microcatheters for extracranial embolization:
i)
Over the wire microcatheters. These are by far the most common and are quite sufficient for nearly all procedures.
(1)
Examples: Excelsior® 10–18 (Stryker Neurovascular, Fremont, CA) Prowler® (Codman Neurovascular, Raynham, MA), Echelon™ (ev3 Neurovascular, Irvine, CA)
c)
Flow-directed microcatheters. These are so flexible distally that they are ideal for catheterizing very small vessels in an atraumatic fashion. However, they are quite flimsy and unstable and are rarely used for extracranial embolization, except in some AVM cases.
i)
Examples: Magic® (AIT-Balt, Miami, FL) Marathon™ or Ultraflow™ (ev3 Neurovascular, Irvine, CA)
d)
Steerable microcatheters. These are the least common and are basically over-the-wire catheters that have the added benefit of a steerable tip of the microcatheter.
i)
Example: Plato™ Microcath (Scientia, Reno, NV).
e)
Two-marker, over-the-wire microcatheters, rather than single-marker catheters, are necessary for the use of detachable coils. The two markers in microcatheters used in detachable coils are always 3 cm apart to determine that the coil is properly deployed. This feature can also be used for calibration and measurements. These two markers may make the distal 3 cm minimally stiffer than one marker catheters, but do not preclude the use of embolic agents other than detachable coils.
3)
Microwire selection
a)
A wide variety of microwires is available, with differing properties such as size, softness, visibility on fluoroscopy, shapeability, and steerability, trackability, and torque control. All microwires suitable for neuro-endovascular procedures are hydrophillically coated to reduce friction.
b)
Wires can have a shapeable distal tip or may come pre-shaped from the manufacturer. Shapeable tips are usually made of platinum, which makes them visible on fluoroscopy.
c)
Sizes of microwires range from 0.008 in. for the tiny Mirage™ (ev3 Neurovascular, Irvine, CA) to a variety of 0.010 in. and even more 0.014-in. wires up to the robust 0.016-in. Headliner™ (Microvention/Terumo, Tustin, CA). Larger diameter wires are available, but are generally a tight fit in commonly used microcatheters and are too stiff for navigation in small vessels.
d)
In general, 0.014-in. wires are used for extracranial embolizations, since they are torquable and will be compatible with most over-the-wire microcatheters.
e)
The Synchro® or Transend® EX (Stryker Neurovascular, Fremont, CA) are very flexible, manoeuvrable wires that work efficiently in most instances.
f)
The authors of this handbook often use the slippery and atraumatic J-tip Headliner™ (Microvention/Terumo, Tustin, CA) in tortuous external carotid branches.
4)
Microcatheter irrigation
a)
Continuous irrigation of the microcatheter as well as the guide catheter with heparinized saline (5,000 U heparin per 500 mL saline) is important. Heparinized saline infusion ensures hydration of the hydrophilic coating on the microwire and minimizes friction.
b)
Meticulous attention to the microcatheter (and guide catheter) RHV throughout the case is necessary to identify thrombus or bubbles, should they appear.
c)
The heparinized saline drip should be periodically monitored to ensure that it is dripping slowly, but continuously, and there is still sufficient fluid in the saline bag to last for the case.
5)
Microcatheter/microwire preparation
a)
Remove the microcatheter from the package and flush the plastic hoop to hydrate the hydrophilic coating.
b)
If necessary, the tip of the microcatheter can be steam shaped over the small mandrel that usually comes packaged with the catheter.
c)
Insert the microwire into the microcatheter, extend the tip of the microwire beyond the tip of the microcatheter, and shape the tip of the microwire with an introducer. Attach the torque device to the microwire.
d)
Tighten the RHV on the microcatheter just enough to prevent leakage of flush and to allow easy use of the microwire.
6)
Microcatheter navigation
a)
Carefully insert the microcatheter into the RHV of the guide catheter and advance it to the distal tip of the guide catheter. Both the Echelon™ and Rebar® (ev3 Neurovascular, Irvine, CA) have a marker on the shaft of the catheter that indicates that the tip is approaching the tip of a 90 cm guide catheter, to limit the need for fluoroscopy up to that point.
b)
Carefully advance the microwire and follow with the microcatheter under roadmap guidance. It is acceptable to advance the tip of the microcatheter without the microwire in straight segments of the vessel.
c)
Carefully guide the microwire around sharp curves and beyond branches by rotating the microwire.
d)
Fixing the microwire in space, the microcatheter can be advanced over the wire and around turns.
e)
To break the friction between microcatheter and microwire, the wire can be gently pulled back and/or rotated.
f)
Monitor the guide catheter position at all times because any resistance to forward motion of the microcatheter will inevitably create backpressure on the guide catheter.
g)
Gently pull back slightly on the microcatheter periodically to remove redundancy.
h)
Also periodically check that the heparinized saline flush lines attached to the guide catheter and microcatheter are dripping and bubble-free.
i)
When the microcatheter reaches the target, remove the microwire and observe the tip of the microcatheter fluoroscopically, since moving the wire can often release stored energy in the microcatheter, causing it to move (usually forward).
j)
Do a microcatheter angiogram to confirm microcatheter positioning and to confirm patency of the microcatheter. Too much resistance during injection can indicate kinking of the microcatheter. Kinking can be resolved by pulling back on the catheter before proceeding further. Forced injection of contrast or embolic material into a kinked catheter can result in catheter rupture, which can be a disaster.
k)
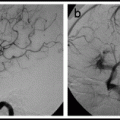
The microcatheter arteriogram shows:
i)
Whether the desired position has been reached
ii)
Whether there are dangerous anastamoses filling
iii)
The flow-rate in order to choose an embolic agent and the injection rate needed
iv)
That there is no sign of contrast exiting the microcatheter proximal to the distal tip. When this happens it is a sign that the microcatheter has been irreparably damaged and must not be used for embolization
l)
Once the microcatheter is in position and a microcatheter angiogram is done, provocative testing may be performed if necessary (see below).
m)
The embolization phase may now begin (also see below).
7)
Flow-directed microcatheter navigation:
a)
Flow-directed microcatheters are commonly used for liquid embolic delivery, usually for treatment of AVMs or AVFs. The high flow state in these conditions greatly facilitates rapid and accurate navigation of the microcatheter to the target. However, in the extracranial circulation, flow rates are less than those in the intracranial circulation, even in hypervascular lesions. Therefore, the flow-directed characteristics of these microcatheters do not assist navigation in the extracranial circulation to any great extent. These catheters can still be used, but technique is virtually the same as that for over-the-wire microcatheters, except that 0.010 or smaller microwires must be used. Again, if Onyx® (ev3, Irvine, CA) is to be used as an embolic agent in the case, a DMSO-compatible microcatheter must be used.
8)
Steerable microcatheter navigation:
a)
Steerable microcatheters are seldom needed for extracranial embolization. They are most appropriate for coil embolization. Their positioning technique is very similar to over-the-wire technique, with a number of idiosyncrasies, given the special steerable characteristics of the catheter. The Plato™ Microcath (Scientia, Reno, NV) is a radically different microcatheter, although it is approved only in Europe at the time of this writing. It is virtually the only true steerable microcatheter available, even it is not readily available at the time of this writing. Further discussions on the use of this microcatheter are in Chaps. 5 and 7.
9)
Provocative testing (see Chap. 6) is done to confirm that the vessel being embolized does not supply dangerous anastamoses to the central nervous system or cranial nerves. Pharmacologic agents such as amobarbital and lidocaine are injected in the vessel prior to embolization and the patient is tested for new signs of neurological dysfunction. Amobarbital injections test for nerve cell body supply in the central nervous system and lidocaine injections test for nerve axons such as cranial nerves.1 Most provocative testing is done on awake patients, although it can be done while the patient is under general anesthesia using neurophysiological monitoring with electroencephalography (EEG), somatosensory evoked potentials (SSEP), brainstem evoked responses (BAER) and/or motor evoked potentials (MEP).2–4 Monitoring is not free from false negatives,2 and the practitioner should never be lulled into a false sense of security even when testing suggests it is safe to embolize, since the pharmacological agents can go preferentially by flow to the abnormal territory. This is especially true in high flow lesions such as AVM or AVF. Careful attention to microcatheter angiograms may be just as sensitive as provocative testing to rule out normal territories at risk. After embolization of a vessel after negative amobarbital and lidocaine testing, a change in flow pattern or visualization or different vessels may be seen after partial occlusion of the vessel. Consider repeating the provocative testing again, and only proceed with further embolization if the provocative testing remained negative.5
8.2.6 Syringe Safety
Many of the procedures discussed in this book require the use of multiple agents in syringes on the procedure table. For example, an embolization procedure that involves provocative testing requires syringes containing local anesthetic, saline flush, contrast, amobarbital, lidocaine, embolic material, etc. It is imperative that these syringes containing different agents are clearly differentiated, one from another. Confusing syringes with anesthetic agents or embolic materials for contrast or saline flush can lead to disastrous results. Use customized, labeled, coloured syringes (Merit Medical, South Jordan, UT) of various sizes and designs for the various materials. Using the same type of syringe for a certain agent at all times and educating new team members to the routine will minimize confusion and avoid mistakes.
8.2.7 Embolization Phase
A variety of embolic agents are available and some are more effective than others. The single most important principle of the selection process is that the operator uses the system with which he or she is most experienced and comfortable.
1)
Embolic material selection
a)
Liquid embolics. The most commonly used intracranial embolic agents.
i)
Cyanoacrylates. (aka glue)
(1)
These are acrylic agents that are in a liquid state and polymerize when they contact hydroxyl ions in blood and are mainly used for intracranial embolization. The most common acrylic agent used in the United States is n-butyl cyanocrylate (n-BCA) Trufill® (Codman Neurovascular, Raynham, MA). Polymerization time can be modified by the addition of oil-based contrast agents such as Ethiodol® (Savage Laboratories, Melville, NY) or glacial acetic acid. Glue tends to cause considerable pain on injection of extracranial vessels, so it is used mainly for spinal embolization, for rare high-flow fistulas in the head and neck for actively bleeding vessels or for direct percutaneous embolization of vascular tumors via needle puncture.
ii)
Precipitated polymer. (aka non-adhesive liquid embolic agent)
(1)
These agents are polymers that are insoluble in blood or water and come dissolved in a non-aqueous solvent. When injected into the vascular system, the solvent disperses and the polymer precipitates to form a solid occlusive agent. Onyx® (ev3, Irvine, CA) is the dominant example of the precipitated polymer and is FDA-approved for use in AVMs. The agent is slowly infused through the microcatheter so is not a particularly useful agent for most applications in the extracranial circulation. The DMSO solvent in the Onyx is locally toxic and causes pain on injection. Another issue with Onyx® is the dark tantalum used to make it radio-opaque which may be visible through the skin if injected in superficial vessels. Consequently it, like the acrylic glue, is also uncommonly used in extracranial procedures.
b)
Sclerosing agents
i)
Sclerosing agents are liquid agents that promote thrombosis and necrosis of the intima to prevent recanalization. Absolute ethanol is medical-grade ethanol that is dehydrated sufficiently to be close to 100% pure ethanol. Absolute ethanol is very thrombogenic and toxic at this concentration. Alcohol should be avoided or only used with utmost caution when treating lesions anywhere near the central nervous system because of this toxicity. It is also extremely painful when injected in vessels in awake patients, and can cause skin necrosis if used in superficial vessels. Consequently, absolute ethanol is usually reserved for attempted palliative embolization for tumours and direct percutaneous sclerotherapy for vascular malformations or tumours in the head and neck. Absolute alcohol should not be confused with Absolut Vodka (Pernod Ricard, Paris, France), which is a trendy alcoholic beverage.
c)
Particles
i)
Particles are the most common agents used in the extracranial head and neck circulation. All particulate agents work best in lesions with a capillary bed, such as tumours. All have a tendency to clog the microcatheter if the particles are too large or injected in too large a quantity. All require a similar technique for their use, and are mixed with contrast and injected via a microcatheter.
(1)
Polyvinyl alcohol foam. (aka “PVA”)
(a)
These are irregularly shaped particles of PVA Examples: Contour® emboli (Boston Scientific, Natick, MA) or PVA Foam Embolization Particles (Cook Medical, Bloomington, IN).
(2)
Spherical emboli
(a)
These particles are manufactured to have a smooth, spherical shape. Examples Spherical Contour SE™ (Boston Scientific, Natick, MA) or Bead Block™ (Terumo Medical, Somerset, NJ) or Embospheres® (Biosphere Medical, Rockland, MA).
d)
Silk suture
i)
Small segments of silk suture can be loaded into a microcatheter and then propelled into the vessel by injecting contrast or saline. Other types of suture material can be used in this fashion, but are less thrombogenic.
e)
Detachable balloons
i)
Detachable balloons are attached to a microcatheter, navigated to the desired site of occlusion, inflated to produce occlusion of the vessel, then detached from the catheter and permanently implanted. They are used on rare occasion in the extracranial circulation for high-flow fistulas or for large vessel occlusion. At the time of this writing, the Goldballoon™ balloon (Balt Extrusion, Montmorency, France), is available in most of the world outside of the United States, but no detachable balloons are currently approved for the North American market.
f)
Pushable coils.
i)
These are platinum coils with thrombogenic fibers that are pushed through the microcatheter with a wire pusher. Examples include Trufill® pushable coils (Codman Neurovascular, Raynham, MA), Hilal and Tornado® Microcoils (Cook Medical, Bloomfield, IN), Fibered Platinum, and Vortx® coils (Boston Scientific, Natick, MA). Small coils such as 2 mm or 5 mm straight coils or 2 mm × 20 mm helical coils can also be propelled through the microcatheter and into the vessel using rapid injections of saline or contrast. Since these are effective in producing vascular occlusion and are inexpensive, they are the most common coils used for extracranial embolization. Still, it is rare that medium sized vessels need occlusion with coils in the extracranial circulation. These coils are best used in the extracranial territories mainly to block anastamotic vessels and prevent particles or liquid emboli from entering the dangerous territory.
g)
Detachable platinum coils.
i)
Detachable coils are rarely used in extracranial embolizations. They are more expensive and not as effective in inducing thrombosis as fibered pushable coils, and are also much slower to deploy. The added precision and security they provide are not usually needed outside the cerebral circulation. They are discussed further in Chap. 5.
h)
Detachable fibered coils
i)
These are a hybrid of the pushable fibered coil and detachable coil. Examples include the Sapphire NXT™ fibered coils (ev3, Irvine, CA). They provide the same features as pushable fibered coils but with added precision. This precision is usually unnecessary in the extracranial circulation.
i)
Stents
i)
Stents are rarely used for embolization in the extracranial circulation such as for the occasional dissecting aneurysm or arteriovenous fistulas that may require stent-assisted coil embolization. The Neuroform™ (Stryker Neurovascular, Fremont, CA) and Enterprise™ (Codman Neurovascular, Raynham, MA) stents may be used in smaller vessels (up to 4.5-mm diameter), but would be used off-label if used in the extracranial circulation. When dealing with AV fistula or pseudoaneurysm arising from larger vessels, the larger self-expanding carotid stents including NexStent® (Boston Scientific, Natick, MA) or Acculink™ (Abbott Laboratories, Abbott Park, IL) can be used for preservation of flow in large or medium sized vessels in extracranial embolization procedures. Coils can be placed in the pseudoaneurysm or on the venous side of the AV fistula, while the stent in the artery prevents herniation of coils into the parent artery. The authors of this handbook have done stent-assisted coiling in cases of wide necked post-traumatic extracranial carotid pseudoaneurysms, or large post-traumatic AVF in the carotid or vertebral artery. It may also be helpful to temporarily inflate a non-detachable balloon, like the Hyperform™ (ev3, Irvine, CA) within the stent during placement of coils or liquid embolic agents in the aneurysm or fistula to provide added assurance that the coils, or other agents do not find their way through the openings of the stent into the parent artery.
(1)
Standard low surface coverage-area intracranial stents like the Neuroform™ or Enterprise™ can sometimes channel flow away from a side-wall aneurysm to induce thrombosis without placing coils. This spontaneous thrombosis after stent placement would not be expected in the case of an AV fistula due to the higher flow conditions. An alternative to this are covered stents, such as the Jostent® (Abbott, Abbot Park, IL). This over-the-wire balloon inflatable covered stent allows rapid occlusion of a fistula without necessarily using coils. The stent is FDA-HDE approved for repair of ruptured coronary vessels 3–5 mm in diameter, and therefore use elsewhere in the body is off-label. Larger vessels (4–7.5 mm) require the Wallgraft™ (Boston Scientific, Natick, MA). This is a self-expanding stainless steel stent-graft is less traumatic than balloon inflatable stents, but requires a large (>8F) sheath. The Viabahn® (W. L. Gore, Flagstaff, AZ) is a heparin coated self-expanding nitinol stent graft that has the added benefit of being MRI compatible. These latter stent-grafts require 7F sheaths for the smaller stents (5 or 6 mm) or 8F for the larger stents (7 or 8 mm). None of these covered stents is FDA-approved for use in the brachiocephalic vessels, but can be life-saving in some cases of active bleeding of a major vessel.6
2)
n-BCA embolization technique. See Chap. 7 for an in-depth discussion of glue injection technique.
a)
Place a flow-directed or over-the-wire microcatheter close to the target and beyond any potential connections to the brain, eye, cranial nerves or spinal cord. Also, avoid getting glue in muscular or cutaneous branches since it may cause considerable pain. Do provocative testing if necessary.
b)
Glue preparation
i)
Set up a separate sterile back table for n-BCA preparation. All personnel working at this table should be free of any contact with saline and should wear eye protection. If a connection comes loose during injection, the glue can spray and stick to whatever it touches.
ii)
Examine the microcatheter angiogram to determine how long the contrast takes to reach the lesion. Rule of thumb: if that time is < 1 s, use at least 70% glue mixture (three parts n-BCA to one part Ethiodol®) is required. Contrast transit time >2 s requires a 50% (one n-BCA to one Ethiodol) or more dilute mixture.
iii)
Tantalum powder greatly increases the radio-opacity of glue, but is not absolutely necessary unless the glue mixture is >70% n-BCA. Tantalum is messy and can clump, and also the pigment may be visible through the skin in superficial vessels, so most practitioners almost never use it in the extracranial circulation.
iv)
Draw up the Trufill® n-BCA (Codman Neurovascular, Raynham, MA) from its tube using a labeled, glue-compatible 3-ml syringe (avoid polycarbonate plastic…it softens).
v)
Draw up the Ethiodol® in a labeled syringe, and add the proper volume to the glue syringe to achieve the desired concentration.
vi)
Have 10–15 labeled 3-ml syringes filled with 5% dextrose solution ready.
c)
Injection technique
i)
Pull back slightly on the microcatheter to remove any slack, and slightly loosen the rotating hemostatic valve so that it just barely prevents back-flow of blood in the guide catheter, without binding the microcatheter too tightly.
ii)
Attach a glue-compatible stopcock directly to the microcatheter. Cook Medical (Bloomington, IN) makes a high-pressure, white nylon plastic one, and three-way stopcocks with Luer lock fittings that hold up well during glue injections.
iii)
Three-way are preferred because they allow a flush syringe of dextrose to remain attached even when the glue syringe is attached. This works well for doing the push technique (see below).
iv)
Thoroughly flush the microcatheter with 5% dextrose solution. Generally, approximately 5–10 ml is sufficient to clear all saline and/or blood from the microcatheter lumen.
v)
As the last dextrose is being injected, close the stopcock to prevent blood backflow into the microcatheter.
vi)
Holding the stopcock upright, fill the Luer-lock connection fully with dextrose.
vii)
Create a blank roadmap to help visualize the glue injection and attach a 3-ml syringe loaded with the prepared glue mixture.
viii)
Swiftly and steadily inject the glue using while watching the roadmap image. Fill the arterial feeder and as much of the nidus as possible.
ix)
Be alert for any signs of reflux of glue back along the catheter, passage of glue into the vein, or reflux of glue from the nidus into other arterial branches feeding the lesion. If any of these conditions is occurring and one is using dilute glue, one might be able to briefly pause the injection, then resume cautiously. Sometimes the glue will find another pathway through the nidus.
x)
The glue injection is quick but controlled. Polymerization occurs within a few seconds. The embolic agent should be deposited in the “safety zone” consisting of AVM nidus and only the artery beyond all normal branches, and vein before other venous inputs beyond the occluded nidus (see Fig. 7.2). If there is any question that the glue is refluxing or going somewhere it shouldn’t, or if finished filling the desired space with glue, stop injecting, aspirate the syringe to create negative pressure in the microcatheter, and quickly, smoothly withdraw the microcatheter completely from the patient and discard it.
xi)
Examine the rotating hemostatic valve of the guiding catheter for any retained droplets of glue, then aspirate and double flush the stopcock, rotating hemostatic valve, and guide-catheter.
xii)
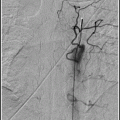
Once the guide catheter is thoroughly inspected and flushed, re-insert it to the arterial territory of interest, and perform a follow-up arteriogram to ensure that the desired result is obtained.
3)
Onyx® embolization technique
a)
Preparation
i)
Have several vials of Onyx® (18 and/or 34) agitating in an automatic mixer for 30 min while performing other parts of the procedure. The Onyx technique is similar to the technique using n-BCA glue except that a dimethyl sulfoxide (DMSO)-compatible microcatheter must be used, such as the Rebar® (ev3, Irvine, CA) or more flexible Marathon™ (ev3, Irvine, CA).
ii)
Note that provocative testing can give a false sense of security since Onyx® can easily find its way into places that may not be predicted by microcatheter angiography or barbiturate injections.
iii)
Confirm proper catheter positioning with a microcatheter angiogram. Select a projection that shows the microcatheter tip and its relationship to any curves in the arterial feeder distal to the catheter tip, any normal branches proximal to it, and whether the tip is wedged.
iv)
Study the microcatheter angiogram to determine the arteriovenous transit time and to visualize the morphology of the target vessels.
v)
Select a pre-mixed viscosity of the agent depending on the size of the feeder and degree of arteriovenous shunting. Big feeders with fast flow need Onyx® 34 and small feeders or slower shunting should be treated with Onyx® 18.
vi)
Draw up 1 ml of DMSO and the Onyx® using the syringes provided in the kit. Agitate the Onyx back and forth if it will not be injected for more than a few minutes to keep the tantalum from precipitating
vii)
Attach the DMSO syringe directly to the hub of the microcatheter and fill the dead-space of the microcatheter (usually 0.2–0.3 ml) with DMSO over 1–2 min.
viii)
Remove the DMSO syringe from the microcatheter, and, keeping the hub upright, fill the hub with DMSO.
ix)
Holding both the catheter hub and Onyx® syringe at 45° to one another, quickly connect the syringe to the hub, and then keep the syringe vertical with the plunger down (see Fig. 7.3). This method keeps a sharp demarcation between the heavier Onyx® in the syringe and lighter DMSO in the hub of the catheter and makes it easier to see radiographically than if the DMSO and Onyx® are allowed to mix together.
b)
Onyx injection technique
i)
Using a blank roadmap, slowly inject the Onyx® at a rate of approximately 0.16 ml/min. Rates of injection >0.3 ml/min risk vascular injury due to DMSO toxicity.
ii)
Continue injecting Onyx® as long as it is flowing forward into desired areas of the abnormal vessels. If it refluxes along the catheter, passes into the proximal part of the vein, or refluxes into other arterial feeders, pause the injection for 15 s, then resume injecting. If the Onyx® continues to flow in the wrong direction, pause again for 15–30 s, then try again. If the Onyx® finds another, more desirable pathway, continue the slow injection.
iii)
Make a new roadmap mask periodically. New roadmaps subtract out the already deposited embolic agent and makes the newly injected material easier to see. Guide catheter angiograms can be done during the Onyx injection to determine if there are still portions of the feeding artery or nidus that could be occluded from this microcatheter position.
iv)
The Onyx® injection requires patience and usually takes at least several minutes.
(1)
Some reflux back along the catheter tip is not a problem, due to the non-adhesive nature of the product. Avoid more than 1 cm of reflux, however, since even Onyx® may glue a microcatheter into the vessel.
(2)
Do not pause the injection for >2 min at a time because the Onyx® may solidify and clog the microcatheter.
(3)
Never try to inject against resistance. A clogged microcatheter may burst if the injection is forced.
v)
When adequate filling of the desired vascular spaces is achieved, or if the Onyx® repeatedly flows in the wrong direction, stop injecting, aspirate back on the syringe, and steadily pull back on the microcatheter, disengage it from the deposited Onyx® and remove it.
vi)
After the microcatheter is withdrawn from the guide catheter, examine the RHV of the guide catheter for any retained droplets of Onyx®, then aspirate and double flush the stopcock, RHV, and guide catheter.
vii)
Do a guide catheter angiogram once the guide catheter is thoroughly inspected and flushed to see what has been accomplished.
4)
Ethanol embolization technique
a)
Ethanol embolization is seldom done for extracranial embolization. It is useful for occasional situations in which the microcatheter tip is very close to the lesion, and for direct puncture of superficial tumours or vascular malformations (see below).
b)
Some operators use Swan-Ganz catheter monitoring for AVM embolization with ethanol, to watch for signs of pulmonary hypertension due to the pulmonary effects of ethanol.
c)
When added to particles, the technique is essentially the same as standard particulate embolization (see below). When used without particles, the technique is more like that for glue.
d)
Be sure to use syringes, stopcocks and microcatheter hubs will not degrade when exposed to ethanol. Often, those that can be used with glue or DMSO will withstand ethanol, but it is wise to test it first. Since ethanol is not an FDA-approved embolic material, manufacturers state that their products are not approved for use with ethanol.
e)
Position the microcatheter and do provocative testing as needed.
f)
Prior to embolizing, do test injections of contrast through the microcatheter to estimate the rate and volume required to fill the vessels that will be treated. If the flow is very rapid, consider placing a coil or two to slow the flow.
g)
Flush the microcatheter with saline because ethanol can cause contrast to precipitate.
h)
Inject the absolute ethanol at a rate similar to the rate that was used for the microcatheter angiogram but use only approximately 50% of the volume of contrast that was used for the microcatheter angiogram.
i)
Wait a few minutes, then repeat the contrast injection. If the target vessels remain patent, inject another small bolus of ethanol, and wait again.
j)
If spasm is seen on repeat test injections, wait until it resolves and decrease the volume of the ethanol bolus.
k)
After a few boluses of ethanol have been given, wait at least 5–10 min between ethanol injections before checking for patency of the vessel.
l)
If there is no change after 20 mL of ethanol, consider placement of additional coils to slow the flow and help the ethanol work, or try a better embolic agent.
m)
Remember that ethanol can work on the endothelium for some time and can also spread through the vessel wall into the adjacent tissues, so it is best to keep the total ethanol volume to a minimum.
5)
Particle embolization technique
a)
Most particles are used in a similar fashion for extracranial embolization.
b)
To avoid major problems with particles clogging the microcatheter, use a larger lumen microcatheter, such as a RapidTransit® (Codman Neurovascular, Raynham, MA).
c)
The microcatheter tip must be close to the lesion being embolized and in a stable position distal to normal branches.
d)
Choose a particle size depending on the size of the vessels in the target lesion. In general, use particles <300 μm in diameter for tumors and particles >300 μm for AVMs.
e)
If there are potential connections to cranial nerves use particles >300 μm.
f)
Mix the particles with 50:50 contrast in saline and draw up the emboli in a labeled 10-ml syringe. This acts as a reservoir for emboli.
g)
The particles should be fairly dilute to limit the risk of clogging the microcatheter.
h)
Attach the syringe to one female connection on a 3-way stopcock and attach a labeled 3-ml Luer-lock syringe to the other female connection. This syringe is used to inject the embolic mixture through the microcatheter.
i)
The stopcock is then attached to the hub of the microcatheter.
j)
The stopcock is turned to connect the 10 and 3 mL syringes, and the contrast/emboli mixture is flushed into the 3-ml syringe and then back into the 10-ml syringe, back and forth several times, to ensure uniform suspension of particles.
k)
The 3-ml syringe is then filled with 1–2 mL of the emboli suspension.
l)
Using a blank roadmap, slowly inject the emboli in small (0.2 mL) increments and ensure that the contrast freely flows from the microcatheter tip.
m)
Increase or decrease the rate of injection, depending on the speed of runoff away from the microcatheter.
n)
Every 3–5 mL of embolic suspension, or sooner if emboli are seen to collect in the hub of the microcatheter, disconnect the 3 mL syringe and reconnect another labeled 3 mL syringe filled with dilute 50:50 contrast.
o)
Gently flush the microcatheter with the contrast under fluoroscopy, remembering that the microcatheter is still full of emboli.
p)
As long as a good runoff of contrast is seen, reconnect the 3-ml embolic syringe, refill it with embolic mixture, and continue to inject emboli.
q)
When the 10 mL syringe is empty, consider obtaining a control superselective angiogram via the microcatheter to see whether the flow pattern is changing.
r)
Especially with AVMs it may require some time and a considerable amount of emboli to occlude a feeder.
s)
If an entire vial of emboli is injected with no change in the flow pattern, consider modifying the flow with a coil or two, or switching to a different embolic agent.
t)
Avoid creating reflux of the embolic mixture back along the microcatheter when injecting. Slow or stop the injections if reflux is seen.
u)
If resistance is encountered during the injections, stop, disconnect the 3 mL embolic syringe and check the hub of the microcatheter. If emboli are bunched up in the hub, it may be possible to rinse them out with a needle or guidewire introducer, then attempt to gently flush with contrast.
v)
If resistance continues, do not attempt to force the emboli through by a forceful injection, and do not use a 1 mL syringe to achieve higher pressures. Forcibly injecting through a microcatheter clogged with particles can cause the microcatheter to rupture and even break into pieces.
w)
When the flow in the feeder is significantly slowed, injections of emboli are stopped.
x)
If more definitive closure of the vessel is needed after particle embolization, a coil or a tiny pledget of Gelfoam may be deposited to finish the job.
y)
Be certain to flush out the microcatheter with contrast or saline before inserting a coil. Particles in the microcatheter can cause the coil to bind in the microcatheter.
z)
Even if the microcatheter seems free of particles, it is best to withdraw and discard the used microcatheter prior to attempting catheterization of another feeder with a new microcatheter.
9)
10)
11)
Get Clinical Tree app for offline access

Stent-graft placement for active bleeding
a)
Begin by obtaining large guide catheter access (a 6F Cook Shuttle is best). Consider placing a second sheath in the opposite femoral artery; this will allow a temporary balloon catheter to be placed in the target vessel to buy time to prepare for stenting. During the access phase, external packing may control the bleeding while the endovascular procedure is underway. In a dire situation, someone may need to manually compress the bleeding site until it is controlled, but their hands will be exposed in the X-ray field and potentially make fluoroscopic imaging difficult.
b)
In the setting of a massive bleeding from trauma or a carotid blow-out or, pretreatment with antiplatelet agents is not advisable. Loading the patient with clopidogril (usually 300–600 mg) only after the stent placement and only after the bleeding has stopped seems to be the most prudent way to handle antiplatelet therapy in this setting.
c)
Size the stent-graft appropriately for the parent artery (usually a little wider than the parent artery) and for the lesion being stented (usually at least 4 mm coverage on either side of the lesion).
d)
Obtain a good roadmap that shows the bleeding site to be treated and also the vessel proximally and distally as much as possible. Consider placing an external radio-opaque marker over the bleeding site to ensure coverage by the stent-graft even if the patient moves.
e)
Advance a microcatheter with a 0.014 in., 300 cm exchange as distal as possible to the lesion being stented. Then remove the microcatheter and leave the exchange-length microwire in place. Take care not to traumatize the bleeding vessel during this process. Always keep the wire tip in view and ensure that it stays in a larger vessel and does not injure the vessel wall.
f)
Advance the stent delivery catheter over the wire, gently pulling back on the wire to make sure the tip remains in a stable position.
g)




Once the stent is in position, remove any slack in the wire and stent delivery catheter. This is critical for obtaining easy and accurate deployment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree