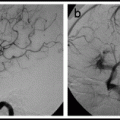
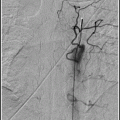
and John P. Deveikis2
(1)
Department of Surgery, Division of Neurosurgery, and Departments of Radiology and Neurology, University of Alabama, Birmingham, AL, USA
(2)
Bayfront Medical Center, St. Petersburg, FL, USA
Abstract
This chapter discusses techniques for intracranial angioplasty and stenting for atherosclerotic arterial stenosis, and angioplasty and intra-arterial drug infusion for cerebral vasospasm after aneurysmal SAH. Position Statement on Intracranial Angioplasty and Stenting for Cerebral Atherosclerosis by the ASITN, SIR, and ASNR: (1) For symptomatic patients with >50% intracranial stenosis who have failed medical therapy, balloon angioplasty with or without stenting should be considered; (2) Patients who have an asymptomatic intracranial arterial stenosis should first be counseled regarding optimizing medical therapy. There is insufficient evidence to make definite recommendations regarding endovascular therapy in asymptomatic patients with severe intracranial atherosclerosis. They should be counseled regarding the nature and extent of their disease, monitored for new neurological symptoms, and have periodic noninvasive imaging at regular intervals of 6–12 months (MRA or CTA) initially, and later with cerebral angiography if warranted. Optimal prophylactic medical therapy should be instituted, which might include antiplatelet and/or statin therapy; and (3) Continued evaluation and improvements in both pharmacological and catheter-based therapies are needed to reduce the possibility of stroke from intracranial atherosclerosis.
This chapter discusses techniques for intracranial angioplasty and stenting for atherosclerotic arterial stenosis, and angioplasty and intra-arterial drug infusion for cerebral vasospasm after aneurysmal SAH.
11.1 Intracranial Atherosclerotic Stenosis
11.1.1 Indications for Intracranial Angioplasty and Stenting
Position Statement on Intracranial Angioplasty and Stenting for Cerebral Atherosclerosis by the ASITN, SIR, and ASNR1:1
1.
For symptomatic patients with >50% intracranial stenosis who have failed medical therapy, balloon angioplasty with or without stenting should be considered.
2.
Patients who have an asymptomatic intracranial arterial stenosis should first be counseled regarding optimizing medical therapy. There is insufficient evidence to make definite recommendations regarding endovascular therapy in asymptomatic patients with severe intracranial atherosclerosis. They should be counseled regarding the nature and extent of their disease, monitored for new neurological symptoms, and have periodic noninvasive imaging at regular intervals of 6–12 months (MRA or CTA) initially, and later with cerebral angiography if warranted. Optimal prophylactic medical therapy should be instituted, which might include antiplatelet and/or statin therapy.
3.
Continued evaluation and improvements in both pharmacological and catheter-based therapies are needed to reduce the possibility of stroke from intracranial atherosclerosis.
11.1.2 Contraindications
1.
Patient inability to have antiplatelet therapy and/or anticoagulation.
2.
Highly calcified lesions or anatomy that prevents endovascular access.
11.1.3 Preprocedure Preparation
1)
Informed consent.
3)
Two peripheral IVs.
4)
Foley catheter.
5)
NPO after midnight or 6 h prior to the procedure except for medications.
6)
Ensure all devices required are available in the angio suite prior to the procedure.
11.1.4 Endovascular Technique
The access phase involves placing a guide catheter in the internal carotid or vertebral artery. The intervention phase includes advancing a microwire across the stenotic lesion, followed by angioplasty with or without stent deployment.
11.1.4.1 Awake or Asleep?
Intracranial angioplasty can be uncomfortable, as stretching and pulling on intracranial vessels are painful. The authors of this handbook use general anesthesia in most cases and the SAMMPRIS protocol required the used of general anesthesia. However, good results have been obtained without anesthesia. Avoiding anesthesia permits continuous neurological surveillance and eliminates anesthesia-associated risks:
1.
Patients who are awake should rehearse the neurological exam on the angio suite table prior to draping. A squeeze toy should be placed in the patient’s hand contralateral to the side being treated.
2.
In a report of 37 intracranial angioplasty and stenting cases without general anesthesia, technical success was achieved in all patients.2 About 61% experienced intraprocedural symptoms that led to some alteration of the interventional technique. Headache was the most common symptom, and, when persistent, signaled the occurrence of intracranial haemorrhage.
11.1.4.2 Access Phase
Patients with intracranial atherosclerosis are also prone to extracranial disease. The reader is referred to Chap. 10, Extracranial Angioplasty and Stenting for a detailed discussion of access techniques and tips for difficult situations. Compared to other intracranial procedures, angioplasty procedures require extra-rigid guide catheter support.
2.
Systemic anticoagulation. Thromboembolic complications can occur during angioplasty, when there is slowing of flow in the parent vessel caused by the guide catheter, or in the target vessel by the microwire or angioplasty balloon.
3.
A loading dose of IV heparin is given (5,000 units or 70 units/Kg. Additional doses of heparin are necessary only for cases lasting several hours.
4.
Protamine on standby – Critical:
a.
A syringe containing enough protamine to reverse the total amount of heparin the patient has received should be kept on the back table for easy access to the operator, should haemorrhage occur:
Dose of protamine required to reverse heparin: 10 mg protamine/1,000 U heparin.
5.
Guide catheter selection and positioning:
a.
The guide catheter should be 90 cm long (and not longer) for use with the Wingspan stent system
b.
Guide catheter support is more important for intracranial angioplasty procedures than most other intracranial interventions. Angioplasty balloons and stents are relatively rigid and difficult to navigate; forward motion of these devices can cause unexpected high amounts of downward-directed force on the guide catheter. Therefore, due caution should be used in guide catheter selection and positioning.
c.
Stiffer guide catheters and positioning of the guide catheter as high as possible helps to maximize support for the intervention.
d.
The catheter tip may slide up and down and rub against the vessel wall with each heart beat; this is to be taken into account when positioning the catheter.
11.1.4.3 Intervention Phase
Once the guide catheter is in position, a good “working view” must be obtained. The working view should be under high magnification and demonstrate the target lesion and distal vessels, and guide the catheter clearly. In most situations, a microcatheter is advanced through the stenotic intracranial vessel over an exchange-length microwire. The purpose of the microcatheter is to facilitate atraumatic and smooth passage of the microwire into a distal vessel; the microcatheter is then removed and the balloon is guided over the microwire into position within the region of stenosis. If stenting is planned, the “pre-dil” balloon is removed and a self-expanding stent (e.g., Wingspan,™ Stryker, Fremont, CA) is deployed. Alternatively, a balloon-mounted stent (e.g., PHAROS™ Vitesse,™ Codman Neurovascular, San Jose, CA) is navigated into position and the stent is deployed.
11.1.4.4 Device Selection
Essential devices for intracranial angioplasty include an exchange-length microwire, a microcatheter, and a balloon. The Wingspan™ Stent System with Gateway™ PTA Balloon Catheter (Stryker, Fremont, CA) was specifically designed for intracranial angioplasty and stenting. It is available on a Humanitarian Device Exemption basis; the use of the Wingspan system currently requires IRB approval. The Wingspan devices and technique are discussed in detail in a separate section below. The PHAROS™ Vitesse™ (Codman Neurovascular, San Jose, CA) balloon-expandable stent is an alternative to the Wingspan stent and is also covered in a separate section below.
1)
Microwires:
a)
“Beefiness,” trackability and torque control are microwire properties that are most important for intracranial angioplasty. A relatively soft distal tip is helpful as well, to minimize the chances of distal vessel vasospasm and perforation.
b)
The authors preferred microwires for most cases:
i)
Transend™ 0.014 in. 300 cm Floppy Tip (Stryker):
(1)
Superior torque control, compared to other microwires.
(2)
Heightened radiopacity makes the tip easy to see on fluoroscopy.
ii.
X-Celerator™ 0.014 in. 300 cm (ev3, Irvine, CA):
(1)
Soft tip, relatively supportive body, very lubricious.
2)
Microcatheters:
a)
A low profile, straight microcatheter, usually of any kind, is sufficient.
b)
The 1.7F Echelon-10 microcatheter (ev3, Irvine, CA) can be pushed through tortuous and stenotic vessels better than other microcatheters.
3)
Angioplasty balloons:
a)
Noncompliant coronary angioplasty balloons are designed to create sufficient radial force to dilate vessels thickened by atherosclerotic plaque. NC: noncompliant:
i)
Selected balloons:
(1)
Gateway™ PTA Balloon Catheter (to be used with the Wingspan stent – see below).
(2)
Maverick2™ Monorail™ balloon Catheter (Boston Scientific, Natick, MA).
(3)
NC Raptor™ balloon catheter (Cordis, Miami, FL).
(4)
Size:
(a)
The diameter of the balloon should correspond to or be smaller than the normal diameter of the vessel; 2.0–2.5 mm diameter balloons are usually appropriate.
(b)
The length of the balloon should be kept to a minimum, to optimize trackability.
4)
Stents:
a)
Wingspan (see below).
b)
Balloon-mounted coronary stents are problematic in intracranial circulation, as they are fairly rigid and difficult to track in tortuous vessels. More importantly, the intracranial arteries, which float freely in CSF, are not surrounded by fibrous connective tissue like coronary arteries and they are more vulnerable to dissection and perforation during deployment of balloon-mounted stents. Relatively high complication rates have been reported with balloon-mounted coronary stents.2–4
i)
If a balloon-mounted coronary stent must be used, cobalt–chromium coronary stents are the easiest to deliver compared to other balloon-mounted stents.2
ii)
The recently-introduced PHAROS™ Vitesse™ (Codman Neurovascular, San Jose, CA) is a balloon-mounted stent specifically designed for the intracranial circulation.
11.1.4.5 Angioplasty Without Stent Deployment
Angioplasty alone is less morbid than angioplasty and stenting with balloon-expandable stent. By itself, angioplasty is a reasonable option for patients with symptomatic intracranial stenosis, particularly because intracranial stenting has not yet been shown to reduce the risk of stroke, and the use of a stent (even the Wingspan system) adds complexity and expense to the procedure. The balloon should be sized to cover the length of the lesion, and the diameter should be ≤ the normal diameter of the vessel. Refer to Wingspan Gateway angioplasty procedure, described below, for a discussion of technique.
11.1.4.6 Wingspan Procedure
The manufacturer of the Wingspan™ Stent System with Gateway™ PTA Balloon Catheter has obtained a Humanitarian Device Exemption from the United States FDA. The system is authorized for use in improving cerebral artery lumen diameter in patients with intracranial atherosclerotic disease, refractory to medical therapy, in intracranial vessels with ≥50% stenosis that are accessible to the system. An IRB approval is currently necessary to use the system.
1.
Devices:
(a)
Guide catheter should be 6F and 90 cm long.
(b)
Microwire. Synchro2™ or Transend™ 0.014 in. Floppy Tip (Stryker, Fremont, CA) is the recommended microwire. Use an exchange-length microwire (300 cm) to maintain microwire access after stent placement in case additional stent placement or post-stent angioplasty is needed.
(c)
Gateway™ PTA balloon catheter:
The Gateway is a modified version of the Maverick2™ balloon catheter, with silicone coating on the balloon and hydrophilic coating on the catheter to facilitate access. Radio-opaque markers on the balloon permit visualization of the proximal and distal ends of the balloon on fluoroscopy.
Available sizes:
Balloon diameters (mm): 1.5, 2.0, 2.25, 2.75, 3.0, 3.25, 3.5, 3.75, 4.0.
Balloon lengths (mm): 9, 15, 20.
Nominal inflation pressure: 6 atm. Rated burst pressure: 12 atm. (14 atm. for 2.25–3.25 mm diameters only).
Size selection:
Plan angioplasty to achieve approximately 80% of normal vessel diameter. For example, for a vessel with a 3.0 mm normal diameter, angioplasty to produce a diameter of about 2.4 mm would be appropriate.
If the target vessel has different diameters proximal and distal to the lesion, size the balloon to the smaller of the two.
Preparation:
Use 50/50 mixture of contrast in heparinized saline.
Prepare the insufflater and attach it with a three-way stopcock and an empty 20 mL syringe to the balloon catheter.
Apply suction to the balloon but do not pre-inflate it.
Continuously flush through the lumen of the balloon catheter with heparinized saline via a stopcock and an RHV.
(d)
Wingspan™ Stent:
The Wingspan is a 3.5F nitinol over-the-wire (OTW) self-expanding stent. The design is very similar to the Neuroform2™ stent (Boston Scientific, Natick, MA); it has four platinum markers at each end for visualization, and is deployed from the delivery microcatheter (called the “outer body”) with the “inner body”; the inner body is analogous to the “stabilizer” device which is used to deploy Neuroform stents.
Available sizes:
Stent diameters (mm): 2.5, 3.0, 3.5, 4.0, 4.5.
Stent lengths (mm): 9, 15, 20.
Size selection:
Select a stent length which extends a minimum of 3 mm on both sides of the lesion.
If the target vessel has different diameters proximal and distal to the lesion, size the stent to the larger of the two.
After deployment, the stent may shorten up to 2.4% in 2.5 mm stents and up to 7.1% in 4.5 mm stents.5
Preparation
The Wingspan system should be flushed with heparinized saline, as indicated in the diagram on the package.
The more flushes, the better. Continuous flushes with heparinized saline should be connected via stopcocks and RHVs to both the Wingspan deployment catheter (the “outer body”) and the inner body.
The tapered tip of the inner body should be loosened slightly, with about 1 mm of space between the spearhead-shaped tip of the inner body and the distal end of the outer body, to allow adequate flushing and prevent “corking,” or binding of the inner body tip to the outer body catheter. During flushing, heparinized saline should be seen dripping from the inner lumen and from between the inner and outer bodies.
2.
Technique:
(a)
Angioplasty:
The Gateway balloon may be taken up primarily, over a nonexchange-length microwire, if the anatomy is favorable. Alternatively, an exchange-length microwire can be advanced into a distal intracranial vessel within a microcatheter, which can be exchanged for the Gateway balloon.
After flushing, advance the balloon catheter over the microwire into the guide catheter. When positioned at the RHV, a marker on the balloon catheter shaft indicates the guide catheter tip. This feature saves fluoro time.
With roadmap guidance, advance the balloon until the balloon markers are across the lesion. Perform a guide catheter angiogram with the balloon in position, to confirm proper positioning.
Inflate the balloon slowly to nominal pressure, at a rate of ∼1 atm./10 s, under fluoroscopy. When the balloon is fully inflated, leave it up for another 10–20 s and then deflate. Do a guide catheter angiogram prior to removing the balloon.
In most cases a single inflation will be sufficient. Occasionally, a second inflation, at a slightly higher pressure (e.g., 8 atm.) is helpful.
(b)
Stent deployment:
Tighten the RHV on the inner body to prevent its migration – and advance the outer body of the Wingspan system over the exchange-length microwire:
The delivery system should be advanced only by grasping the outer body, to avoid inadvertently advancing the inner body and prematurely deploying the stent.
Advance the outer body slightly past the region of stenosis.
Using the marker bands to identify the position of the stent, advance the inner body just proximal to the stent.
Pull back on the outer body, to bring the outer body tip into position just past the region of stenosis; this should be the final maneuver prior to stent deployment.
Deploy the stent by holding the inner body in a stable position with the right hand, while, with the left hand, slowly withdraw the outer body.
Do not attempt to change the position of the stent during deployment.
Once the stent is deployed, bring the deployment system into the proximal part of the vessel, or into the guide catheter, while leaving the microwire in position. Do a guide catheter angiogram.
3.
Gateway and Wingspan tips.5:
Get Clinical Tree app for offline access

(a)
Do not over tighten the RHV around the balloon catheter shaft.
(b)
If the balloon is difficult to inflate, remove it and use another device.
(c)
If the balloon watermelon-seeds (i.e., slips forward or backward during inflation)
Apply gentle traction to the balloon catheter during inflation, to stabilize the balloon and prevent it from migrating distally during inflation, or
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree