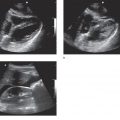
General Surgery Applications
Over the past three decades, abdominal sonography has become increasingly utilized as a diagnostic tool for surveying hepatobiliary, vascular, urologic, or GYN disorders. With progress in the resolution of scanning devices, it has also been used for the evaluation of various acute GI abnormalities. In the emergency setting, the focused assessment with sonography for trauma (FAST) examination has been widely performed by growing numbers of nonradiologist physicians, such as emergency physicians and surgeons, and accepted as a rapid and appropriate screening tool for trauma. In the same way, bedside abdominal sonography has been increasingly utilized for surveying the acute abdomen. The operator-dependent nature of ultrasonography, however, may limit the application of the examination in the emergency or acute care setting. In many hospitals, the difficulty in providing 24-hour ultrasonography service has been a major factor in preventing ultrasound from becoming a primary imaging modality. However, it is quite important to utilize the advantages of sonography to improve patient evaluation in the emergency or acute care setting.
This chapter discusses practical applications of sonography for the acute abdomen, especially for surgical emergencies associated with this presentation.
 CLINICAL CONSIDERATIONS
CLINICAL CONSIDERATIONS
The evaluation of acute abdominal disorders begins with a careful history and physical examination. When required, the clinical findings may be supplemented by laboratory tests or conventional plain radiographs. Plain radiography may show some significant findings, such as pneumoperitoneum and bowel dilatation, but unsatisfactorily, it shows nonspecific findings in a significant number of patients. The development of high-resolution CT and ultrasonography has greatly facilitated the identification of pathology in many patients with an acute abdomen.
CT is an excellent imaging modality to evaluate not only intraperitoneal disorders but also retroperitoneal abnormalities. CT has a greater specificity than plain radiography. Multiple-detector CT (MDCT), which has taken the place of single-detector CT in recent years, shows three-dimensional, high-quality images of the viscera and presents detailed structures of acute abdominal abnormalities. Both plain radiography and CT are noninvasive, but are contraindicated in pregnant patients. The level of irradiation for CT may be more than a hundred times of that in plain radiography.
In contrast, sonography does not expose patients to ionizing radiation and is noninvasive, readily available, repeatable at the bedside, and less expensive than CT. It has been accepted as a useful imaging modality for hepatobiliary, CV, urologic, or GYN disorders. In addition, it has been demonstrated that sonography is applicable and accurate for acute GI disorders such as acute appendicitis, acute colonic diverticulitis, intussusception, and bowel obstruction. Abdominal sonography, however, has some disadvantages such as difficulty in visualizing abnormalities in patients who are obese or who have excessive bowel gas.
The operator-dependent nature of ultrasonography is one factor influencing the reliability of point-of-care ultrasound performed by nonradiologist physicians. Indeed, the clinical applications and results of point-of-care ultrasound are influenced by the clinical experience, skill, and interest of the clinician. In several European countries and Japan, however, point-of-care ultrasound performed by nonradiologist physicians has been accepted as a rapid and useful screening tool for evaluating the acute abdomen as well as for abdominal trauma. Physicians who are well trained to perform point-of-care ultrasound will significantly improve patient evaluation, initial treatment, selection of further diagnostic modalities, and timely consultation of surgeons or GI specialists.
Color and power Doppler imaging have been applied to a variety of GI disorders, vascular disorders, and various tumors in the abdomen. In addition, contrast-enhanced ultrasound has been used for differentiating ischemic intestinal disorders. These modalities may enable visualization of vascularity in the affected segment of the intestine. In the emergency setting, however, they have been of limited use because of the uncertain efficacy and the difficulties in application. At present, they are not essential to the diagnosis of inflammatory disorders, but are complementary to differentiating between inflammation and ischemia.
 CLINICAL INDICATIONS
CLINICAL INDICATIONS
In general, the clinical indications for performing a point-of-care ultrasound examination in this setting are
 Acute abdominal pain and peritonitis
Acute abdominal pain and peritonitis
 Intractable nausea and vomiting
Intractable nausea and vomiting
 Abdominal distention or mass
Abdominal distention or mass
 Unexplained shock or sepsis
Unexplained shock or sepsis
All patients who have been diagnosed with an acute abdomen on the basis of clinical findings are candidates for the examination. Table 11-1 lists the common etiologies of an acute abdomen.
 TABLE 11-1. COMMON ETIOLOGIES OF AN ACUTE ABDOMEN
TABLE 11-1. COMMON ETIOLOGIES OF AN ACUTE ABDOMEN
Hemorrhage
GI perforation
Bowel obstruction
Inflammatory disorder
Circulatory impairment
HEMORRHAGE
Active intraperitoneal or GI bleeding is a life-threatening etiology for which rapid diagnosis and treatment are required. If patients are hemodynamically unstable, adequate resuscitation is the first priority. Rapid assessment for the approximate site of hemorrhage (intraperitoneal or GI) should be made on the basis of clinical findings. The use of sonography for patients with massive hematemesis is limited in the emergency setting because they should be referred for emergency endoscopy. Patients who are hemodynamically unstable without GI bleeding should be urgently examined for intraperitoneal hemorrhage. In this setting, abdominal sonography is very useful and reliable for the evaluation of intraperitoneal hemorrhage. It can be utilized during the resuscitation of the unstable patient in the emergency setting.
Intraperitoneal Hemorrhage
Rapid detection of free intraperitoneal fluid is essential since intraperitoneal hemorrhage may be severe enough to produce hypovolemic shock. For this purpose, the FAST examination is beneficial. As described in Chapter 5, “Trauma,” sonography has been recognized as a rapid, sensitive, and specific diagnostic modality for detecting free intraperitoneal fluid.1,2 Appropriately trained nonradiologist physicians, such as emergency physicians and surgeons, can accurately perform and interpret abdominal sonography for free intraperitoneal fluid. Plain radiographs are insensitive and inappropriate for the early recognition of intraperitoneal hemorrhage since radiographic signs for the accumulation of peritoneal fluid, such as widening of the paracolic gutter or a “dog’s ear” appearance, require a large amount of peritoneal fluid. While CT is very useful for detecting intraperitoneal hemorrhage and retroperitoneal hematoma, it is inappropriate for hemodynamically unstable patients.
In making a decision for surgical exploration, it is important to detect the presence and the amount of intraperitoneal hemorrhage even if primary lesions are not identified. As abdominal sonography can be used to estimate not only the amount but also the rate of intraperitoneal hemorrhage through serial examinations, it will supplement clinical findings in evaluating whether the hemorrhage is active or not.
Common sites where free intraperitoneal fluid accumulates are Morison’s pouch, the rectovesical pouch, the pouch of Douglas, and bilateral subphrenic spaces. A small amount of free intraperitoneal fluid may be seen only between bowel loops. A large amount of free intraperitoneal fluid can be seen above the bowels, located adjacent to the anterior peritoneum. On sonographic images, hemoperitoneum appears anechoic with coarse internal echoes as the blood is clotted. Bloody or purulent ascites or peritoneal fluid containing intestinal contents also may be shown as having similar images. It is not very difficult, however, to differentiate hemoperitoneum from ascites on the basis of a careful history and physical examination. If required, paracentesis (guided by ultrasound) can be applied for the definitive diagnosis of hemoperitoneum.
The pathology causing intraperitoneal hemorrhage can be evaluated with abdominal sonography. While CT remains the gold standard for detecting specific intraabdominal pathology, it is beneficial to utilize point-of-care ultrasound for this purpose, especially with unstable patients. Common causes of intraperitoneal hemorrhage are rupture of a hepatoma, abdominal aortic aneurysm, ectopic pregnancy, or ovarian lesion. Abdominal sonography can be performed as a rapid screening tool for detecting such specific lesions as hepatoma and abdominal aortic aneurysm. Early recognition of these etiologies is beneficial for selecting further imaging tests and strategizing on treatment options, such as immediate surgery or interventional radiology procedures. In young women who present with hypotensive shock and associated lower abdominal pain, disorders, such as rupture of an ectopic pregnancy or ovarian bleeding unrelated to pregnancy, should always be taken into consideration. Although transabdominal sonography may demonstrate only nonspecific findings, massive hemorrhage warrants immediate surgical intervention.
GI Hemorrhage
Patients with an upper GI hemorrhage generally present with varying degrees of hematemesis or melena. However, some may present with only complaints of epigastric pain or with unexplained shock. Primary causes of upper GI hemorrhage are listed in Table 11-2. Patients suspected of having massive GI hemorrhage should be referred for emergency endoscopy, which makes it possible to identify the bleeding source in up to 90% of cases of upper GI hemorrhage. Diagnostic modalities such as plain radiography, ultrasonography, CT, and GI contrast studies do not contribute to the diagnosis of acute GI hemorrhage. When appropriate, point-of-care ultrasound may be used to detect adjunct findings such as liver cirrhosis and splenomegaly. Abnormalities of the gastroduodenal wall occasionally may be shown with sonography in cases of gastric cancer, peptic ulcer, or acute gastric mucosal lesion.3,4
 TABLE 11-2. PRIMARY CAUSES OF UPPER GI HEMORRHAGE
TABLE 11-2. PRIMARY CAUSES OF UPPER GI HEMORRHAGE
Duodenal ulcer
Gastric ulcer
Hemorrhagic gastritis
Esophageal or gastric varices
Mallory–Weiss syndrome
In cases of massive lower GI hemorrhage, direct endoscopic evaluation may be disturbed by a large amount of blood and stool in the colon. Furthermore, at times, the causes of lower GI hemorrhage originate in the small bowel. For these reasons, emergency angiography or scintigraphy is reserved for patients in whom colonoscopy is unsuccessful in locating the bleeding source. Abdominal sonography can be used as a screening tool to evaluate intra-abdominal abnormalities suggesting a bleeding source (e.g., colon cancer and ischemic colitis) and its adjunct findings (e.g., liver cirrhosis, bowel obstruction, or abscess formation).
GI PERFORATION
GI perforations are serious disorders requiring rapid diagnosis and treatment. Since they may be severe enough to produce septic or hypovolemic shock, rapid decision making for urgent laparotomy is crucially important. The initial diagnosis is generally made on the basis of clinical symptoms and signs of peritonitis and then supplemented by plain radiographs demonstrating pneumoperitoneum. Plain radiographs, however, may not always show pneumoperitoneum in cases of GI perforation, and are useless in detecting underlying etiologies. The incidence of pneumoperitoneum appreciated on conventional radiographs was reported 80–90% in cases of gastroduodenal perforation but only 20–30% and 30–50%, respectively, in cases of small bowel and large bowel perforation.5,6 Moreover, in the elderly, signs of peritonitis on physical examination may be obscured and laboratory tests may show a normal WBC. These clinical and radiographic features may cloud the diagnosis of GI perforation in elderly patients. Consequently, any delay in making a decision for urgent laparotomy may lead to further deterioration in the clinical status of the patients, especially in cases of large bowel perforation. To avoid such delay in the diagnosis and treatment, conventional plain radiography should be supplemented with other diagnostic modalities, which include CT, sonography, contrast studies, or endoscopy. CT is very sensitive for demonstrating not only pneumoperitoneum but also ectopic gas in the retroperitoneal space. CT may demonstrate a very small pneumoperitoneum that is not appreciated on conventional plain radiography.7
Abdominal sonography is not as sensitive as plain radiography for demonstrating pneumoperitoneum. It may be valuable, however, in complementing plain radiographs by rapidly identifying pneumoperitoneum in the supine patient.8–12 Subphrenic free air can be identified as an echogenic line with posterior reverberation artifacts on the ventral surface of the liver. It should be discriminated from gas in the GI lumen or the lung to avoid a false diagnosis. Hyperventilation may interfere with the examination for visualization of free air. Hepatodiaphragmatic interposition of the colon also may cause subphrenic gas echoes.
As for the underlying pathology, point-of-care ultrasound can be applied for the evaluation of specific lesions. It may detect a primary lesion, such as acute colonic diverticulitis, colon cancer, or an acute duodenal ulcer, and secondary abnormalities, such as free peritoneal fluid, a localized abscess, or paralytic ileus. Upper GI perforation is not very difficult to diagnose on the basis of clinical and radiographic findings. If required, emergency endoscopy can be adopted for identifying gastroduodenal lesions. Therefore, it is not essential to detect images of a peptic ulcer or gastric cancer by sonography or CT. As a screening tool available at bedside, however, abdominal sonography may occasionally demonstrate a gastroduodenal ulcer or gastric cancer as hypoechoic wall thickening.3,4
The strategies for treatment of a peptic ulcer, which is the leading cause of upper GI perforation, have been changed in recent years. Nonoperative treatments using antiulcerative agents have been successful in selected patients with a perforated duodenal ulcer. This new option in treating perforated peptic ulcers may influence the use of diagnostic modalities. A patient with a perforated duodenal ulcer can be a candidate for nonoperative treatments when signs of peritonitis are localized in the right upper quadrant. In this setting, consequently, pneumoperitoneum itself is not considered to be an absolute indication for immediate surgery. Serial examinations with sonography can be used as follow-up studies to evaluate the accumulation of peritoneal fluid or occurrence of any other abnormalities when nonoperative treatments are adopted for a perforated duodenal ulcer.
On sonographic images of GI perforation, free intraperitoneal fluid often contains gray level echoes inside an anechoic space in the pelvis or Morison’s pouch, or adjacent to intestinal loops. The image is regarded as showing turbulent, purulent, or feculent peritoneal fluid. Gas echoes may be occasionally identified as echogenic spots inside an anechoic space. Although the nature of peritoneal fluid cannot be ascertained strictly on ultrasound, such sonographic images can be helpful in making a decision for surgical intervention when pneumoperitoneum is not identified.
BOWEL OBSTRUCTION
Bowel obstruction is a common etiology of acute abdomen. The clinical picture of a patient with bowel obstruction varies depending on location, form, etiology, and degree of the obstruction. Thus, strategies for treatment should be carefully determined on the basis of clinical findings, laboratory tests, and imaging methods. Generally, plain radiography is conventionally used as an initial imaging method when bowel obstruction is considered. It serves to confirm the distribution of gaseous dilated bowel and the approximate site of obstruction. However, it is widely known that plain radiography cannot reliably differentiate strangulation from simple obstruction and is useless to demonstrate causative lesions for bowel obstruction.13,14
For years, the application of sonography for bowel obstruction has been regarded as inappropriate and unreliable because of the significant artifact arising from GI gas. This misconception has prevented not only radiologists but also surgeons and emergency physicians from utilizing sonography for the evaluation of bowel obstruction. With progress in the resolution of scanning devices, however, abdominal sonography has become more popular for the evaluation of GI diseases.15–17 Ultrasound’s role in recognizing fluid-filled distended bowel was reported in the literature during the latter half of 1970s.18,19 Fleischer and coworkers first introduced sonographic patterns of distended, fluid-filled bowel both in vivo and in vitro in 1979.18 Since the latter half of 1980s, abdominal sonography has gained increasing popularity for the evaluation of bowel obstruction in Japan and Germany. Some studies have shown the usefulness of abdominal sonography for demonstrating a radiograph-negative small bowel obstruction, and for differentiating between a small bowel obstruction and a paralytic ileus.20–23 In the 1990s, the use of sonography for the differentiation between strangulation and simple small bowel obstruction was reported.24–26 Ogata and colleagues introduced the usefulness of sonography in identifying radiograph-negative large bowel obstruction, and Ogata and Mateer prospectively demonstrated that initial point-of-care ultrasound was as sensitive as, and more specific than, plain radiographs for the diagnosis of bowel obstruction in an ED setting.27,28
The pathophysiologic appearances of bowel obstruction are characterized primarily by the accumulation of fluid in the GI tract proximal to the obstruction.29 Along with further progression of bowel obstruction, the bowel loops become distended with accumulated fluid in the lumen (Figure 11-1). In addition, the bowel wall may be thickened with interstitial edema, and free fluid may accumulate in the peritoneal cavity. Taking these features into consideration, abdominal sonography as well as CT may be appropriate and applicable to the diagnosis of bowel obstruction because it is superior to plain radiography in visualizing accumulated fluid. Furthermore, real-time sonography can provide a dynamic view of intestinal peristalsis, which is not recognized with CT. These advantages of real-time sonography have made a revolutionary progress in the diagnosis of bowel obstruction, especially in the early recognition of strangulated small bowel obstruction.
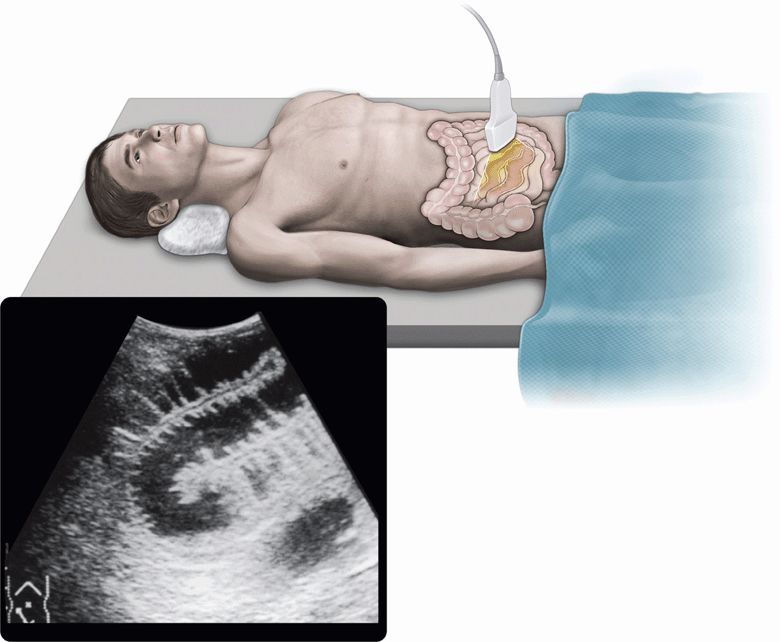
Figure 11-1. Fluid-filled dilated small bowel.
Strangulated small bowel obstruction involves compromise of blood supply to the strangulated loop of bowel and requires early surgical intervention. It is difficult to recognize the early stages of strangulation because of lack of reliable criteria.13 Difficulty in making the early diagnosis of strangulation has resulted in a recommendation of early surgical intervention. While this strategy seems logical in reducing delays in surgical repair, it increases the number of surgical cases for non-strangulated obstruction that could have been relieved without operative therapy. However, in order to safely elect nonoperative treatment, the exclusion of strangulation is essential. Ogata and associates reported that abdominal sonography was useful in revealing the presence of strangulation that was not suspected by clinical judgment. Abdominal sonography was also useful for excluding the presence of strangulation in patients with simple obstruction who were clinically suspected of having strangulation.24 According to their reports, the sensitivity and specificity of sonography for strangulation were 90% and 92%, respectively, in the study of 231 patients with small bowel obstruction by adhesions. The use of sonography to differentiate strangulation from simple obstruction may permit earlier operative intervention for strangulation and allow wider use of non-operative management for simple small bowel obstruction.
When abdominal sonography is applied for the evaluation of bowel obstruction, it is clinically important to analyze the following points in each case:
1. To identify the evidence of mechanical bowel obstruction
2. To locate the level of obstruction
3. To differentiate strangulation from simple obstruction
4. To evaluate the etiology of bowel obstruction.
5. To estimate the severity of bowel obstruction.
6. To survey the whole abdomen for other abnormalities
Mechanical Bowel Obstruction Versus Ileus
The clinical manifestations of a mechanical bowel obstruction depend on the level of the obstruction (proximal or distal small bowel or large bowel) and the blood supply to the affected loop of bowel (simple or strangulated obstruction). The evidence of a mechanical bowel obstruction is confirmed by demonstrating a distinct point of transition between dilated proximal bowel and collapsed distal bowel with an imaging modality such as plain radiography, sonography, or CT. In contrast, the diagnosis of ileus is based on the absence of such a distinct point of transition along with a clinical presentation consistent with ileus. Abdominal sonography, as well as plain radiography and CT, can be used for the differentiation of a mechanical bowel obstruction versus an ileus.20,28,30 In the early stage of ileus, slightly dilated small bowel loops (<25 mm wide in diameter) are often recognized on ultrasound. Gas echoes, which are more dominant than fluid collection inside the bowel, are featured in the sonographic images of ileus. Also, other abnormalities suggesting the primary etiology of ileus may be shown on ultrasound. In the advanced stage of ileus, real-time sonography may occasionally show fluid-filled dilated bowel loops without peristaltic activity.
Small Bowel Obstruction Versus Large Bowel Obstruction
Initially, abdominal sonography was used in suggesting the diagnosis of small bowel obstruction in patients with atypical plain radiographs, such as a “pseudotumor” appearance or a totally “gasless” abdomen, and also in demonstrating an intussusception in patients with an abdominal mass suspected of the entity. Real-time point-of-care ultrasound can be used as an initial imaging method for the evaluation of small bowel obstruction.21,23,25 According to the report by Ogata and associates, GI gas interfered with ultrasound examinations in only 3 of 231 patients with small bowel obstruction by adhesions.24
As for patients clinically suspected of having a large bowel obstruction, plain radiographs are routinely used as the initial imaging modality as it serves to confirm the diagnosis and locate the obstruction in the majority of the cases. However, plain radiographs may show an isolated small bowel dilatation but no gaseous colonic dilatation in approximately 15% of patients with large bowel obstruction.14,27 In such cases, it is difficult to differentiate a large bowel obstruction from a small bowel obstruction on plain radiographs alone. The use of sonography for the diagnosis of large bowel obstruction has not yet been fully evaluated because of the belief that accumulated gas in the colon interferes with the examination. Indeed, it is difficult to evaluate the gaseous distended colon with sonography. In cases of large bowel obstruction, however, abdominal sonography often reveals dilated colon filled with dense spot echoes, which seem to represent feculent, liquid contents including small bubbles of gas. In one study, abdominal sonography provided a diagnosis of large bowel obstruction in 33 of 39 patients with this condition, and proved useful in detecting radiograph-negative colonic dilatation that was occasionally seen in patients with large bowel obstruction proximal to the splenic flexure.27
Strangulation Versus Simple Obstruction
Adhesive bands most commonly cause strangulated small bowel obstruction. The strangulated closed loop may occasionally be shown as a “pseudotumor” appearance on plain radiographs. Sonography is effective in demonstrating the closed loop filled with fluid. Real-time sonography also can provide a dynamic view of peristalsis in the obstructed loops.
The sonographic criteria for simple small bowel obstruction include the presence of dilated small bowel proximal to collapsed small bowel or ascending colon, and the presence of peristaltic activity in the entire dilated proximal small bowel. The peristaltic activity is appreciated as peristalsis of the bowel wall or to-and-fro movements of spot echoes inside the fluid-filled dilated small bowel.
The criteria for early strangulation include (1) the presence of an akinetic dilated loop, (2) the presence of peristaltic activity in dilated small bowel proximal to the akinetic loop, and (3) rapid accumulation of intraperitoneal fluid after the onset of obstruction. An established strangulation is recognized by asymmetric wall thickening (>3 mm) with increased echogenicity in the akinetic loop, or a large amount of peritoneal fluid containing scattered spot echoes indicating bloody ascites.
The presence of intraperitoneal fluid is not specific for strangulation, but the quantitative evaluation of intraperitoneal fluid is helpful in differentiating strangulation from simple obstruction. The presence of an akinetic dilated loop is an ominous sign, but should be judged carefully with several minutes’ observation or serial observations in order to avoid overlooking intermittent peristaltic activity. In some cases, when the obstruction becomes prolonged or anticholinergic drugs are administered, peristaltic activity may cease in dilated intestinal loops without circulatory impairment. In addition, peristaltic activity may occasionally be observed in a viable state of an early or partially strangulated loop.
Color or power Doppler imaging may demonstrate reduced blood flow within the strangulated loop in hemorrhagic necrosis, but does not surpass B-mode ultrasound in the early recognition of strangulated obstruction.
Specific Etiologies of Obstruction
Abdominal sonography offers the advantage of providing additional information about specific etiologies of obstruction that is not obtained with plain radiographs. Although adhesions obstructing the small bowel cannot be visualized, ultrasound can image the specific etiologies of small bowel obstruction, which are listed in Table 11-3. With the exception of intussusception and incarceration of external hernia, these specific etiologies are relatively rare but should be considered.
 TABLE 11-3. ETIOLOGIES OF SMALL BOWEL OBSTRUCTION SEEN ON ULTRASOUND
TABLE 11-3. ETIOLOGIES OF SMALL BOWEL OBSTRUCTION SEEN ON ULTRASOUND
Cecal carcinoma
Intussusception
External hernias
Inflammatory bowel diseases
Small bowel tumors Afferent loop obstruction following Billroth gastrectomy
Gallstone ileus
Intussusception is a common etiology of bowel obstruction in children but relatively rare in adults, accounting for only about 5% of all intussusception cases and 1–3% of adult patients with bowel obstruction. Unlike in children, the causative lesion can be identified in more than 80% of adult patients. The most common cause is a polypoid tumor of the small bowel. Ileocolic intussusception is the most common form (>70%), followed by enteroenteric and colocolic intussusception. Plain radiographs rarely define the intussusception as a mass of soft tissue density, and show no evidence of bowel obstruction in the acute stage. In contrast, abdominal sonography can present the characteristic appearances of intussusception. The cross-sectional image is well known as the “multiple concentric ring sign” or “target sign.”31,32 The multilaminar structure also can be demonstrated in the long-axis planes. It is very rare, however, to demonstrate the causative lesion itself (i.e., tumor or diverticulum) with sonography. The sonographic appearance of bowel obstruction may not yet be established when the diagnosis of intussusception is obtained.
Incarceration, which is a common complication of external hernias, produces a bowel obstruction and impairs the blood supply to the entrapped bowel segment. Among the common external hernias including external inguinal hernia, internal inguinal hernia, femoral hernia, and abdominal incisional hernia, incarceration occurs most frequently in cases of femoral hernia. The common hernia content is small bowel. The diagnosis is not difficult to make on the basis of a careful physical examination in most cases. If physical examination findings are equivocal, abdominal sonography can be used to demonstrate an incarcerated hernia, showing an entrapped bowel segment in the abdominal wall. However, incarcerated obturator hernia is a rare entity among the external hernias, and hardly noticed as a mass because it is located deep in the femoral region. It occurs occasionally in thin, elderly females, and is usually diagnosed as a small bowel obstruction by clinical symptoms, physical examination, and plain radiographs. Abdominal sonography can demonstrate an entrapped bowel segment medial to the femoral artery and vein, and posterior to the pectineus muscle in the femoral region. CT of the pelvis demonstrates an entrapped bowel segment between the pectineus muscle and the obturator externus (or internus) muscle.
As for the etiologies of large bowel obstruction, obstructing colon carcinoma, which is by far the most common cause of large bowel obstruction, may be detected as an irregular-shaped hypoechoic mass with echogenic core inside or a localized circular wall thickening. Intraluminal tumor obstructing the lumen may be occasionally demonstrated with sonography. Ogata and associates reported that sonography demonstrated the obstructing lesion in 14 of 35 patients with primary or metastatic colorectal carcinoma.27 Even when the obstructing lesion is not visualized, detecting the associated lesions such as metastatic liver tumors would be useful in making the diagnosis. In volvulus of the sigmoid colon, however, sonography shows only vast gas echoes that spread beneath the abdominal wall because the twisted and obstructed colon loop is markedly distended with excessive gas. Plain radiography is diagnostic of this entity by presenting the classic “coffee bean” sign. In volvulus of the entire small bowel, a rare entity in the Western countries, sonography may show fluid-filled dilated loops with mural thickening and intraperitoneal fluid. Peristaltic activity dwindles as the intestinal infarction progresses.
INFLAMMATORY DISORDER
Various kinds of inflammatory disorders are included in the etiologies of acute abdomen. Abdominal sonography can be used for evaluating the site, extent, or severity of the inflammatory disorder by visualizing interstitial edema or hemorrhage, and peritoneal fluid. Segmental wall thickening of the bowel may be demonstrated in inflammatory GI disorders such as appendicitis, diverticulitis, infectious enterocolitis, ischemic colitis, or Crohn’s disease.17 Also, wall thickening of the gallbladder can show the severity of acute cholecystitis, and the echogenicity of the pancreas varies according to the degree of interstitial edema or hemorrhage in acute pancreatitis.
Color and power Doppler imaging can be used to evaluate vascularity in the affected segment of the intestine or the gallbladder and may present complementary findings in the diagnosis of inflammatory diseases. Without careful interpretation of B-mode sonograms, however, their utility would be of limited value.
Acute Appendicitis
Acute appendicitis is the most common cause of the acute abdomen in Western countries. The diagnosis is straightforward in most patients who present with typical clinical symptoms and signs. It is not uncommon, however, to face difficulties in making a diagnosis of appendicitis in patients who have an equivocal presentation. Conventional radiographs present nonspecific findings, such as regional bowel dilatation, in most cases of acute appendicitis. The most specific finding on plain radiographs is the presence of a calcified appendicolith, which is noted in about 10% of adults with appendicitis.
However, abdominal sonography has been increasingly used for the diagnosis of acute appendicitis, and consequently, is considered to be useful for (1) direct visualization of the inflamed appendix (Figure 11-2), (2) assessment for the degree of inflammatory changes, (3) identification of abscess formation or free peritoneal fluid, (4) differentiation from other acute abdominal disorders, and (5) application to pregnant patients.
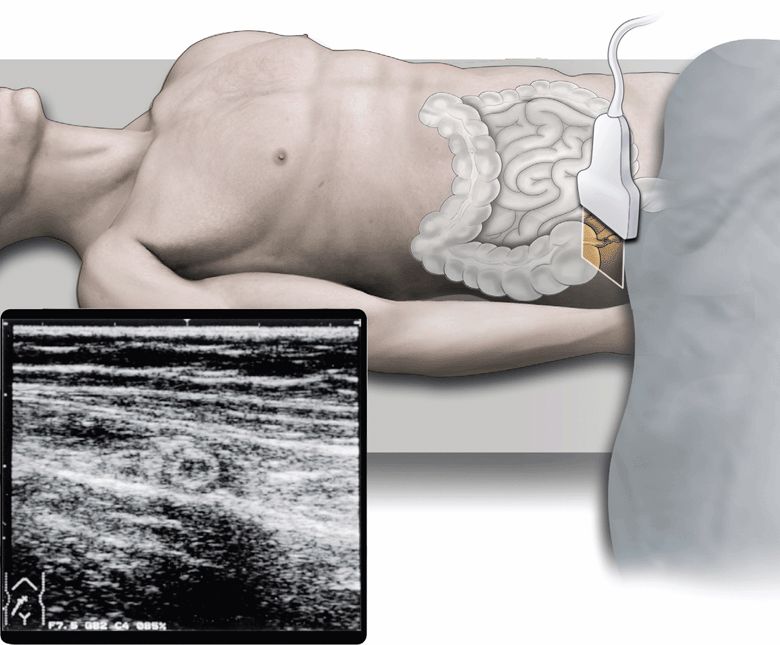
Figure 11-2. The cross-section of inflamed appendix.
Since the first report that high-resolution ultrasound with a graded compression technique was successful in visualizing the abnormal appendix in a high percentage of cases, many physicians have adopted the technique and confirmed high diagnostic accuracy of the technique for acute appendicitis.33–39 The sensitivity and specificity of graded compression sonography in experienced hands were reported to be 76–90% and 90–98%, respectively. In the United States, abdominal CT scan is commonly utilized for evaluating patients with possible appendicitis; its accuracy for confirming or ruling out appendicitis has been reported to be 93–98%.
The accuracy of sonography is operator dependent. Practically, inexperienced operators will face difficulties in obtaining a high accuracy rate in the diagnosis of acute appendicitis.40,41 The most important reason for a false-negative study is overlooking the inflamed appendix. Dilated bowel loops due to an associated ileus may obscure the appendix. Optimal images may not be obtained because of the inability to achieve adequate compression of the right lower quadrant. This is caused by severe pain or marked obesity. False-negative studies may also occur in patients with retrocecal or perforated appendicitis. A false-positive diagnosis can be made if a normal appendix is mistaken for an inflamed one or if a terminal ileum is confused with an enlarged inflamed appendix. With adequate training and enough experience, however, nonradiologist physicians can obtain an acceptable accuracy rate in comparison with experienced operators.42–45
Sonography is more sensitive for the detection of an appendicolith than plain radiographs, and has been reported as detecting intraluminal fecaliths in up to 30% of cases.46 In general, a normal appendix (about 6 mm or smaller) can rarely be visualized by graded compression sonography although some investigators have reported that in the majority of patients a normal appendix can be identified in experienced hands.46,47
Acute appendicitis may present in various stages at the time of diagnosis: catarrhal, phlegmonous, gangrenous, or perforated accompanying pericecal abscess or purulent peritonitis. Abdominal sonography can be used to evaluate the pathologic severity of acute appendicitis by delineating the layer structure of thickened appendiceal wall. In cases of catarrhal or phlegmonous appendicitis, a swollen appendix maintains the mural lamination. In contrast, focal loss of the layer structure is often observed in patients with gangrenous appendicitis. A pericecal abscess can be demonstrated as fluid collection with a thick, noncompressible wall. With a pericecal abscess secondary to perforated appendicitis, it may be quite difficult to identify the gangrenous appendix itself. Even if an inflamed appendix is not detected, identifying an abscess or free peritoneal fluid in the pelvis or the pericecal region can be valuable for surgeons to make a decision for urgent exploration. However, it is still controversial whether surgical intervention or conservative treatment with antibiotics should be adopted in the early stage of appendicitis. In general, sonographic findings should be correlated with both clinical and laboratory findings to determine an indication for surgery.
Acute appendicitis in pregnant women can be rather difficult to diagnose because of the deviated location of the appendix and equivocal presentation. Abdominal sonography can be applied for the evaluation of appendicitis in pregnant patients. In this setting, it is important to take the deviated location of the appendix into consideration.
Sonography is also useful for establishing an alternative diagnosis in patients examined with suspicion of appendicitis.48 The spectrum of differential diagnoses includes mesenteric lymphadenitis (particularly in children), right-sided adnexal pathology in young women, enterocolitis, diverticulitis, Crohn’s disease, cholecystitis, and colon cancer.
Acute Colonic Diverticulitis
The prevalence of colonic diverticulosis increases with age. Acute colonic diverticulitis is a relatively common etiology of the acute abdomen in elderly patients, although approximately 80–90% of all diverticula remain asymptomatic for life. The rectosigmoid colon is the most frequently involved segment in acute colonic diverticulitis. Diverticulitis in the ascending colon and cecum is less frequently involved, but seen in younger patients and frequently in the Asian countries. It is occasionally misdiagnosed as acute appendicitis since it is accompanied with the symptoms or signs similar to the entity.
Plain radiographs are of little value in obtaining direct findings of acute diverticulitis, but may demonstrate pneumoperitoneum or ileus in complicated cases. The use of contrast barium enema for demonstrating the extent of the disease is limited to the cases of clinically mild diverticulitis because it is hazardous in cases of possible colonic perforation. Water-soluble contrast enema is safe and available in complicated cases, although the quality of images is inferior to barium contrast enema.
Abdominal sonography can be applied for the initial evaluation of possible diverticulitis. Both the sensitivity and specificity of sonography for this etiology was reported more than 80% when the examination was performed by experienced operators.49–52 Abdominal sonography may reveal additional findings such as pericolonic abscess or free intraperitoneal fluid in complicated cases. CT is better in demonstrating not only colonic diverticula but also extracolonic complications, including pericolonic or pelvic abscess, free perforation, or colovesical fistula.
Acute Pancreatitis
Acute pancreatitis is defined as an inflammation of the pancreas associated with typical abdominal complaints and elevated serum pancreatic enzymes, and may be classified according to the clinical picture, etiologic factors, or pathologic changes. The clinical course ranges from a mild, benign process to a severe, fulminant process that may lead to fatal outcomes. The two most common etiologic factors are alcoholism and biliary stone disease, although up to 10–30% of patients with acute pancreatitis may present without a history of either.
The pathologic forms are classified generally as edematous and necrotizing pancreatitis. Edematous pancreatitis is characterized by interstitial edema and mild pancreatic and peripancreatic inflammation, and accounts for 80–85% of cases. The mortality rate is less than 2%. Necrotizing pancreatitis is characterized by interstitial hemorrhage, fat necrosis, extensive extrapancreatic infiltration, and suppuration. Bacterial infection occurs in up to 40% of patients with necrotizing pancreatitis and is gradually manifested within several weeks after the onset of pancreatitis. On the whole, necrotizing pancreatitis is a far more severe form of acute pancreatitis that often requires hemodynamic support and mechanical ventilation, and leads to severe complications and mortality rates of 10–40%.53,54 Although it is clinically important to distinguish patients with either edematous or necrotizing pancreatitis in terms of therapy and prognosis, it is rather difficult at the early stage of their clinical course. Quantitative assays of serum pancreatic enzymes may be useful for the diagnosis of acute pancreatitis, but the degree of the enzymes does not correlate with the severity of the disease. Plain radiographs may show nonspecific findings such as the “sentinel loop sign,” “colon cut-off sign,” or a generalized ileus, but are of little use in evaluating acute pancreatitis.
Direct imaging of the pancreas with CT or sonography may provide morphologic information to establish the diagnosis of pancreatitis and its complications.54–56 In general, CT is clearly superior to ultrasound in demonstrating complex extrapancreatic involvement as well as contour irregularities or focal changes in the pancreas, and used as the gold standard imaging modality for acute pancreatitis and its complications. Contrast CT is recommended to discriminate necrotizing pancreatic tissues from other parts. It is also advantageous in locating fluid collections to specific anatomic compartments. Pancreatic abscess is often associated with extensive, ill-defined multicompartmental changes. However, it is difficult to distinguish pancreatic abscess from uninfected necrosis or fluid collections. CT-guided fine-needle aspiration can be used to make an early diagnosis of pancreatic abscess.
Ultrasound examinations are frequently disturbed by excessive GI gas caused by an accompanying ileus, especially in cases of severe pancreatitis. Therefore, the primary role of point-of-care ultrasound is to evaluate the biliary tree for gallstone disease as a remediable cause. Sonographic diagnosis of choledocholithiasis or significant dilatation of the common bile duct may obviate the need for invasive diagnostic procedures. The secondary role is to evaluate peripancreatic fluid collections or intraperitoneal fluid. Extrapancreatic fluid collections are most commonly detected in the superior recess of the lesser sac and the anterior pararenal space in cases of acute pancreatitis. Fluid collections are generally visualized as anechoic or hypoechoic images on ultrasound.
Acute peripancreatic fluid collections resolve with conservative therapy in 70–90% of the cases. The remaining fluid collections persist long enough (at least 6 weeks) to develop a fibrous wall, and then are called pancreatic pseudocysts. Pseudocysts may develop in association with chronic pancreatitis, or after pancreatic surgery or trauma. Uncomplicated small pseudocysts (smaller than 6 cm) may allow persistent observation, but larger pseudocysts should be drained by surgical, endoscopic, or percutaneous means to reduce the risk of complications, which include secondary infection, rupture, and hemorrhage.53,54 Serial examinations with CT or sonography can document the gradual development of pseudocysts. The advantage of sonography is lower cost for follow-up studies. On sonographic images, pancreatic pseudocysts are generally visualized as cystic masses of various sizes, which are well defined by adjacent organs and a visible capsule. However, small well-defined cystic masses should be examined with color Doppler scanning to exclude a pancreatic pseudoaneurysm, which may occasionally develop 2–3 weeks after the onset of severe pancreatitis.
Abdominal sonography can also be used for the initial survey of acute pancreatitis. The echogenicity of the pancreas generally decreases in acute pancreatitis as a result of interstitial edema. In some patients, however, the echogenicity is normal or increased. The echogenicity of the pancreas compared to the liver has been found to be increased in 16% and normal in 32% of patients with acute pancreatitis.55 The variability may be caused by pancreatic hemorrhage, necrosis, or fat saponification. Enlargement of the pancreas is also variable in acute pancreatitis, and significant individual variations are recognized in pancreatic dimensions. Therefore, enlargement of the pancreas is of limited value for the diagnosis of acute pancreatitis. Echogenic pancreatic masses may suggest the progress of necrotizing pancreatitis, and should be confirmed with both contrast and noncontrast CT for the definitive diagnosis.
Acute Cholecystitis
Since acute cholecystitis may lead to serious complications such as sepsis, pericholecystic abscess, or bilious peritonitis secondary to gallbladder perforation, immediate surgery is often required. Therefore, it is critically important to make a rapid and accurate diagnosis of acute cholecystitis and to determine the indication for surgical intervention. Abdominal sonography is a rapid and reliable technique for establishing or excluding the diagnosis of acute cholecystitis, even though sonographic findings should be always correlated with clinical and laboratory findings (see also Chapter 10, “Hepatobiliary”).
There are three important indirect signs to establish the diagnosis of acute cholecystitis.57,58 Gallstones are the prime etiologic factor since approximately 90% of cases with acute cholecystitis develop as a complication of cholelithiasis. The identification of impacted stones in the gallbladder neck or cystic duct is highly specific for acute calculous cholecystitis, although sonography may be unable to detect a small impacted gallstone in a few cases of calculous cholecystitis. Biliary sludge along with the absence of gallstones in the expanded gallbladder can be identified in cases of acalculous cholecystitis. The most specific sign of acute cholecystitis is the “sonographic Murphy’s sign,” which corresponds to the spot of maximum tenderness directly over the gallbladder (Murphy’s sign is elicited with focal tenderness over the gallbladder with inspiratory arrest). According to one study, 99% of patients with acute cholecystitis had calculi and a positive sonographic Murphy’s sign.58 In cases of acalculous cholecystitis, however, focal tenderness over the gallbladder may be difficult to obtain.
Thickening of the gallbladder wall to more than 3 mm is another sign for acute cholecystitis, although it is not specific as long as the wall maintains a distinct three-layer structure with a hypoechoic band surrounded by two hyperechoic lines. Irregular sonolucent layers in the gallbladder wall may be indicative of more advanced cholecystitis. The presence of asymmetric thickening of the gallbladder wall or intraluminal membranes parallel to the gallbladder wall may be identified in patients with acute gangrenous cholecystitis. Localized pericholecystic fluid collection may be caused by gallbladder perforation and abscess formation. The site of perforation may occasionally be visualized as a defect in the gallbladder wall. These sonographic findings can be indicative of the need for immediate surgery.
CIRCULATORY IMPAIRMENT
Ischemic bowel disease requires prompt treatment, either by surgical exploration or interventional radiology. It is challenging to demonstrate ischemia at the early stage of the disease entity. Consequently, delay in diagnosis may lead to intestinal necrosis in a significant number of patients with ischemic bowel disease.
In theED, acute mesenteric ischemia and ischemic colitis should always be considered in the elderly patient who presents with acute abdomen or unexplained shock. Acute mesenteric ischemia is caused mainly by embolism or thrombosis of the superior mesenteric artery (SMA). Nonocclusive mesenteric ischemia may develop secondary to hypoperfusion of the intestine in cases of serious illness, including heart failure, sepsis, or shock. Superior mesenteric vein thrombosis may develop secondary to abdominal surgery, trauma, acute pancreatitis, or coagulopathy.
Superior Mesenteric Artery Occlusion
Acute mesenteric artery occlusion is notoriously difficult to diagnose early in its clinical course, and subsequently often results in delayed surgical intervention in a number of cases. Patients present with sudden onset of abdominal pain, diarrhea, or vomiting. However, the symptoms are nonspecific and there may be a striking disparity between the severity of symptoms and the lack of direct physical findings. Progressive signs of shock may be apparent in the initial stage. Therefore, it is clinically important to suspect an SMA occlusion when elderly patients present with nonspecific abdominal symptoms. An SMA embolism may develop in cases of mitral valve disorders or atrial fibrillation. SMA thrombosis is related to atherosclerotic disorders.
Routine abdominal sonography does not provide specific findings in cases of an SMA occlusion. In the initial stage, fluid-filled dilatation of the small bowel is minimal. Peritoneal fluid and mural thickening in the small bowel without peristaltic activity are nonspecific, but suggest the possibility of acute mesenteric ischemia. Compared with strangulated small bowel obstruction, dilatation of the small bowel is not recognized as significant. In the advanced stages, a large amount of peritoneal fluid can be demonstrated. The application of color Doppler ultrasound is limited to detecting an occlusion of the main trunk of the SMA.59 However, it is not so easy to confirm the etiology by examination because excessive GI gas caused by an accompanying ileus disturbs the examination. Segmental mesenteric arteries cannot be demonstrated with color Doppler ultrasound.
Contrast and noncontrast CT can demonstrate decreased enhancement in the vascular territories of the SMA, and may directly demonstrate an occluding thrombus within the SMA, pneumatosis of the bowel wall, or gas in the portal vein in conjunction with peritoneal fluid, bowel wall thickening, or dilatation of fluid-filled loops of the small bowel. When SMA occlusion is suspected, immediate angiography should be applied for the definite diagnosis or nonsurgical intervention.
Ischemic Colitis
Ischemic colitis is characterized by the abrupt onset of crampy abdominal pain and diarrhea that often contains blood. Since the clinical features are nonspecific and few symptoms may be present initially, it is especially important to consider this entity in elderly patients. Unlike small bowel ischemia, most cases of colonic ischemia are not associated with a visible arterial occlusion. The pathophysiology is believed to relate to decreased perfusion of the colon wall due to peripheral vasoconstriction (e.g., in cardiac failure), sepsis, or hypovolemia. Age-related atherosclerotic disease is a predisposing factor. The most common site of involvement is the distal colon within the vascular territory of the inferior mesenteric artery. The proximal colon to the splenic flexure area (the so-called “watershed zone”) may be involved. The effects of ischemia range from reversible mucosal ischemia to transmural infarction. Most cases of ischemic colitis are resolved conservatively with medical treatment. Urgent laparotomy is required in complicated cases with gangrene or perforation of the affected colon.
While colonoscopy remains the primary method to evaluate patients with clinically suspected ischemic colitis, abdominal sonography can be used as an initial diagnostic method for the entity. In the acute phase, circumferential hypoechoic wall thickening is demonstrated in the affected segment of the colon. The laminar structure of the wall is visualized as less distinct on ultrasound exams using a high-frequency transducer. As routine sonography cannot reliably differentiate inflammatory changes from ischemic changes, color Doppler ultrasound should be applied for the differentiation.60–62 Mural blood flow is diminished in the affected segment of ischemic colitis. Both routine sonography and color Doppler scanning can be used for a follow-up study of ischemic colitis. In cases of reversible mucosal ischemia, wall thickening is gradually reduced and mural blood flow increases approximately in 1 week. In cases of transmural infarction, sonography may show rapid accumulation of peritoneal fluid. CT scan can be used for the same purpose and is more sensitive for complications such as perforation or abscess formation.
 ANATOMICAL CONSIDERATIONS
ANATOMICAL CONSIDERATIONS
FREE INTRAPERITONEAL FLUID
The site of accumulation of intraperitoneal fluid is dependent on the position of the patient and the etiology that causes free fluid to accumulate. In the supine patient, intraperitoneal fluid in the pelvis or Morison’s pouch is most easily detected by sonography.
STOMACH
The stomach can be identified by the subcostal or subxiphoid scanning. The gastric antrum is generally located posterocaudally to the left lobe of the liver. The cardia is identified posterior to the lateral segment of the liver. In the emergency setting, the proximal stomach is difficult to clearly delineate due to the significant artifact arising from gas in the stomach.
DUODENUM
The duodenal bulb is located medially to the gallbladder, posterior to the liver, and anterior to the pancreatic head. The inferior vena cava is another landmark located posterior to the duodenal C-loop.
SMALL BOWEL
Generally, the jejunum is located in the left upper and mid abdomen and the ileum is located in the right mid and lower abdomen. The small bowel cannot be traced continuously by sonography.
LARGE BOWEL
The ascending colon is easily demonstrated anterior to the right kidney in the right flank. The transverse colon can be identified caudally to the gastric antrum in a sagittal plane. The descending colon can be demonstrated anterior to the lower pole of the left kidney in the left flank. The sigmoid colon may be difficult to examine. The rectum can be demonstrated posterior to the uterus or prostate.
APPENDIX VERMIFORMIS
The psoas muscle and the external iliac artery and vein are important anatomic landmarks when searching for the appendix. The position of the appendix is highly variable. The most common position is caudal to the cecum and terminal ileum, followed by a retrocecal position. Other less common positions are deep within the pelvis, lateral to the cecum, and mesocecal.
PANCREAS
The pancreas is easily located by its vascular landmarks. In transverse planes, the pancreas lies posterocaudal to the left lobe of the liver and crosses over the aorta and the inferior vena cava. The splenic vein is a useful landmark for identifying the pancreas as it runs along the posterior surface of the pancreas. In sagittal planes, the pancreatic body is located posterior to the gastric antrum and the left lobe of the liver, and anterior to the splenic vein and the SMA. The pancreatic head lies anterior to the inferior vena cava and caudal to the portal vein. The pancreatic duct runs along the length of the gland, and is best imaged in the pancreatic body. The duct can be frequently visualized as a tubular structure with reflective walls with maximum diameter up to 2 mm. The anteroposterior diameters of the head and body are, in general, less than 3 cm and 2 cm, respectively. Wide normal variations are noted in pancreatic dimensions, and tend to decrease with age. The normal pancreas is homogeneous with the echogenicity greater than or equal to the adjacent liver.
 GETTING STARTED
GETTING STARTED
In the emergency and acute medical care setting, a rapid, focused inspection and systematic survey of the entire abdomen are required for obtaining useful information. Among a number of factors that influence the accuracy of point-of-care ultrasound, the most critical is the clinician’s experience, which includes not only the technique of scanning but also the knowledge of the clinical and pathologic findings in acute abdominal disorders.
Positioning of the patient during the point-of-care ultrasound examination is important for obtaining optimal images. Place the patient in the supine position. To avoid interference by gas echoes, place the patient in the semilateral, lateral, or semierect positions. Oblique or coronal planes are more frequently used than sagittal or transverse planes, especially in patients who have a bowel obstruction or ileus. Perform the standard examination using a sweeping motion with a convex transducer (3–5 MHz). Use a higher-frequency (>7 MHz) transducer for delineating the laminar structure of the appendix, GI wall, or specific lesions of the abdominal wall.
Focused assessment with sonography is recommended according to the purposes, situations, and various levels of examiners. Avoid an unnecessary, time-consuming examination for obtaining unfocused findings so as not to delay patient treatment. Inexperienced operators should begin with the survey for free intraperitoneal fluid, and then proceed to the focused assessment for gallstone-related disorders, hydronephrosis, abdominal aortic aneurysm, dilated small bowel, or large abdominal tumors. Intraperitoneal fluid is the first priority to evaluate in cases of acute abdomen as well as trauma, since the presence, amount, location, and internal echoes of accumulated intraperitoneal fluid are correlated with the etiology or severity of acute abdominal disorders.
 TECHNIQUE AND NORMAL ULTRASOUND FINDINGS
TECHNIQUE AND NORMAL ULTRASOUND FINDINGS
FOCUSED ASSESSMENT WITH SONOGRAPHY FOR THE ACUTE ABDOMEN
For cases of an acute abdomen, perform a rapid inspection for free intraperitoneal fluid in a manner similar to the FAST examination (see Chapter 5, “Trauma”) (See Videos 5-1–5-6). Use the right intercostal and coronal views to examine for free intraperitoneal fluid in Morison’s pouch and the right subphrenic space. From these views, the right kidney and the right lobe of the liver can be inspected briefly. Use the left intercostal and left coronal views to examine for free intraperitoneal fluid in the left subphrenic space and the splenorenal recess. From these views, the spleen and the left kidney can be inspected briefly. Then, use the pelvic (sagittal and transverse) views to examine for free intraperitoneal fluid in the pelvis. From these views, the bladder and the prostate or uterus can be inspected briefly.
Next, perform a focused inspection for acute abdominal disorders in a systematic fashion. Determine the areas to be examined first according to the clinical findings, but survey the entire abdomen to exclude less suspicious disorders in the differential diagnosis. Subphrenic free air is best visualized on the ventral surface of the liver with right intercostal scanning. The patient may be placed in the semilateral position elevating the right flank.
SCANNING PROCEDURES FOR GASTRODUODENAL ABNORMALITIES
In scanning the epigastric region, demonstrate the gastric antrum anterior to the pancreatic body and posterocaudal to the left lobe of the liver (Figure 11-3). Visualize the proximal stomach posterior to the left lobe of the liver by using the liver as an acoustic window (Figure 11-4), although the examination is often disturbed by a significant artifact arising from gas in the stomach or the transverse colon. Identify the distended proximal stomach filled with liquid contents medially to the splenic hilum with left intercostal or coronal scanning.
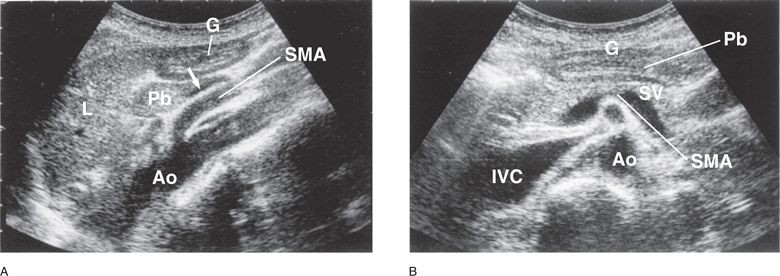
Figure 11-3. Gastric antrum. (A) In an epigastric sagittal plane, the cross section of gastric antrum is visualized anterior to the pancreatic body and caudal to the left lobe of the liver. The pancreatic body is located anterior and cephalad to the splenic vein (arrow) and the SMA. (B) In a transverse plane, the gastric antrum is demonstrated anterior to the pancreatic body. G = gastric antrum, L = left lobe of the liver, Pb = pancreatic body, Ao = aorta, IVC = inferior vena cava, SMA = superior mesenteric artery, SV = splenic vein.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 CLINICAL CONSIDERATIONS
CLINICAL CONSIDERATIONS CLINICAL INDICATIONS
CLINICAL INDICATIONS ANATOMICAL CONSIDERATIONS
ANATOMICAL CONSIDERATIONS GETTING STARTED
GETTING STARTED TECHNIQUE AND NORMAL ULTRASOUND FINDINGS
TECHNIQUE AND NORMAL ULTRASOUND FINDINGS COMMON ABNORMALITIES
COMMON ABNORMALITIES COMMON VARIANTS AND OTHER ABNORMALITIES
COMMON VARIANTS AND OTHER ABNORMALITIES PITFALLS
PITFALLS CASE STUDIES
CASE STUDIES