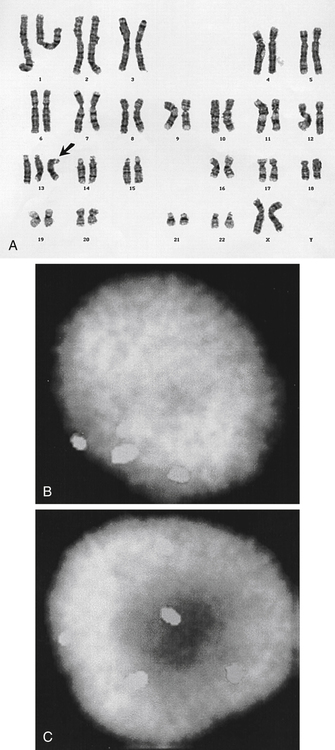
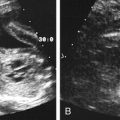
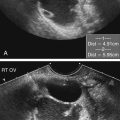
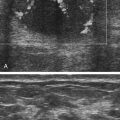
• Describe the method of noninvasive biochemical testing for assessment of fetal aneuploidy. • List the methods of invasive testing for chromosomal analysis. • Describe the anomalies associated with an increased nuchal translucency. • List the sonographic markers associated with chromosomal anomalies. • Describe the sonographic findings associated with trisomies 13, 18, 21; Turner syndrome, and triploidy. Multiple prenatal screening options are available to help identify pregnancies that may be at an increased risk for fetal aneuploidy. Down syndrome, or trisomy 21, is the most common chromosome abnormality seen in live births. A trisomy 21 pregnancy is detected more frequently in older women than in younger women, and the use of maternal age alone helps detect approximately 30% of fetuses with trisomy 21.1 Incorporation of serum (i.e., blood) screening with early sonographic evaluation has improved detection rates greatly. Offering screening options to all women increases the chance of detecting abnormalities earlier and allows time for counseling and referral for women in need of a targeted sonographic evaluation (level II) or diagnostic testing (e.g., amniocentesis).With advances in ultrasound technology, it is now possible to assess fetuses in greater detail at an earlier gestational age. PAPP-A is a first-trimester biochemical marker produced by the placental trophoblast and found in maternal serum. Pregnancies affected by trisomy 21 and trisomy 18 have a decreased PAPP-A. Free β hCG is a glycoprotein hormone derived from the placenta that peaks at 8 to 10 weeks’ gestational age and then decreases to a plateau. Free β hCG is increased in pregnancies with trisomy 21 and decreased in pregnancies with trisomy 18 (Table 24-1). The use of the two analytes in conjunction with maternal age and sonographic evaluation of nuchal translucency (NT) has an estimated detection rate of 85% for trisomy 21.2 Additionally, it has been noted that an unexplained low PAPP-A is associated with an increased frequency of adverse obstetric outcomes.3,4 TABLE 24-1 First-Trimester Multiple Marker Screening The sequential screen and the integrated screen are two tests currently available that combine the first-trimester screen with second-trimester analytes to provide better detection rates for trisomy 21and trisomy 18. The sequential screen incorporates the first screen with a second blood draw at 15 to 21 weeks of gestation that looks at AFP, hCG, uE3, and DIA. A preliminary result is given 1 week after the first blood draw with the final result available 1 week after the second blood draw. The sequential screen has a detection rate of trisomy 21 and trisomy 18 of approximately 90%.4 The integrated screen also follows the two blood draws protocol and uses the same analytes as the sequential screen, but no preliminary result is given, and the final result is available 1 week after the second blood draw. The integrated screen has a slightly better trisomy 21 detection rate (approximately 92%); the trisomy 18 detection rate remains at 90%. It is helpful to have the patient meet with a genetic counselor to review all of the testing options available; this allows patients to make an informed decision regarding which test would best meet their needs. Four blood tests are currently available for fetal genetic screening in the second trimester: maternal serum AFP test, triple screen, quadruple (quad or tetra) screen, and penta screen. Blood work should be drawn between 15 and 21 weeks of gestation, but guidelines and requirements may vary among laboratories. The maternal blood work and information regarding fetal gestational age and number, maternal age, ethnicity, family history, maternal weight, and diabetic status are evaluated to give a fetal risk assessment for neural tube defects, trisomy 21, and trisomy 18. These results are expressed as a numeric value or odds ratio. The odds ratio assigns a risk value for open neural tube defect, Down syndrome risk, and trisomy 18 risk. Current second-trimester screening cannot provide a risk assessment for trisomy 13, Turner syndrome, or congenital heart defects. Table 24-2 lists the draw times and detection rates and Table 24-3 lists the analytes assayed for each maternal serum screening test discussed. TABLE 24-2 Draw Times and Detection Rates TABLE 24-3 Maternal serum AFP is a biochemical test that measures the amount of AFP within the maternal serum. AFP is an oncofetal protein produced by the fetal liver and yolk sac. Maternal serum AFP results are expressed in multiples of the median (MoM), using a normal range of 0.25 to 2.50. Some laboratories use 2.0 as their upper MoM cutoff. Maternal serum AFP was the first prenatal screening test developed in the late 1970s for the detection of neural tube defects. Shortly afterward, it was recognized that fetuses with trisomy 21 had lower levels of serum AFP (<0.74 MoM). Elevated levels of AFP have been associated with open neural tube defects, open ventral wall defects, multiple gestations, placental abnormalities, and numerous fetal structural defects and disorders. In the presence of a normal targeted sonogram, an unexplained elevation in the maternal serum AFP has been associated with preeclampsia and an increased frequency of adverse obstetric outcomes.4 The triple screen is a biochemical test that evaluates AFP, hCG, and uE3 in the maternal serum. uE3 is a steroid hormone produced by the fetal liver and placenta. Low levels of uE3 have been seen in association with Down syndrome, Smith-Lemli-Opitz syndrome, and steroid sulfatase deficiency.4 The combination of elevated hCG and decreased AFP and uE3 yields an increased risk for trisomy 21 in that pregnancy. The triple screen has a detection rate of trisomy 21 of approximately 70%.4 When hCG, AFP, and uE3 all are decreased, there is an increased risk for trisomy 18 in that pregnancy. An increased frequency of adverse obstetric outcomes is associated with an unexplained elevation in maternal serum AFP (>2.5 MoM) or hCG (>3.0 MoM), or both, or a decreased level of maternal serum AFP (<0.25 MoM) or uE3 (<0.5 MoM), or both.3,4 The quad screen adds DIA to the analytes used in the triple screen. DIA is produced in the placenta and increases the detection rate of Down syndrome to 75% to 80%. An unexplained elevation in DIA (≥2.0 MoM) is associated with an increased frequency of adverse obstetric outcomes.4 In contrast to the serum markers used in the triple test, DIA values are independent of gestational age. However, the quad screen includes hCG, AFP, and uE3, so it is imperative that an accurate gestational age be assigned. An analyte pattern of decreased maternal serum AFP and uE3 with an elevation of hCG and DIA yields an increased risk for Down syndrome. In pregnancies with trisomy 18, AFP, uE3, and hCG all are decreased. DIA does not contribute to the detection of trisomy 18. The penta screen uses the four previous maternal biochemical markers in conjunction with hyperglycosylated hCG. Some laboratories refer to hyperglycosylated hCG as invasive trophoblast antigen. This addition increases the detection of trisomy 21 slightly to 83%.5 The penta screen is the newest screen and not yet as proven as the quad screen. Chromosome analysis from CVS can be used to identify genetic abnormalities, although they may be confined just to the placenta. When mosaicism is identified, an amniocentesis may be necessary to determine whether the abnormal chromosome pattern is also in the fetus. In addition, CVS does not evaluate AFP and cannot screen for open neural tube defects. Fetal limb-reduction abnormalities have been associated with CVS performed before 10 weeks’ gestational age. Fetal loss rates are similar to loss rates associated with second-trimester amniocentesis.6,7 An alternative to CVS is early amniocentesis, which may be performed between 12 and 15 weeks’ gestational age. Although early amniocentesis alleviates the risk of an additional invasive procedure for placental mosaicism, failed attempts may occur when tenting of the amniotic membranes results from the lack of fusion of the amnion to the chorion. This tenting of the membranes does not allow the needle to enter the amniotic cavity to draw the amniotic fluid. Testing of amniotic fluid AFP is impossible until after 15 weeks’ gestational age. The fetal loss rate may vary with gestational age, although studies have found the loss rate to be increased over that of second-trimester amniocentesis.7 Early amniocentesis has also been associated with talipes equinovarus.6 Early and midtrimester amniocentesis procedures are performed in a similar manner. Midtrimester amniocentesis to rule out genetic abnormalities is usually performed between 15 and 18 weeks’ gestational age. The current fetal loss rate is stated to be 0.5%.7 The procedure usually includes a sonogram to screen for abnormalities and check gestational age, fetal number, and cardiac activity. An amniocentesis is performed transabdominally under direct ultrasound guidance. Results of the chromosomal analysis are usually available in 7 to 14 days and are considered the “gold standard” for detection of chromosomal abnormalities. Conventional chromosomal analysis of the amniotic fluid may require 2 weeks to culture and evaluate the chromosomes (Fig. 24-2, A). This time could add additional stress to patients who are at increased risk for aneuploidy based on maternal serum screening, advanced maternal age, or sonographic findings. Fluorescence in situ hybridization (FISH) allows for limited analysis of uncultured amniotic fluid or chorionic villi, with results available within 24 hours. The FISH assay typically evaluates for numeric abnormalities of chromosomes 13, 18, 21, X, and Y by adding a probe labeled with fluorescent dye to the fetal cells. The number of colored signals (Fig. 24-2, B and C; see Color Plate 31) represents the number of copies of select chromosomes. The information obtained is more limited than with a cultured analysis, but the results regarding the most common aneuploidies can be obtained more quickly. A conventional cultured analysis should be completed to confirm the findings.
Genetic Testing
Maternal Screening Options
Maternal Serum Screening
First-Trimester Screening
Down Syndrome
Trisomy 18 and Trisomy 13
Free β hCG
↑
↓
PAPP-A
↓
↓
NT
↑
↑
Second-Trimester Screening
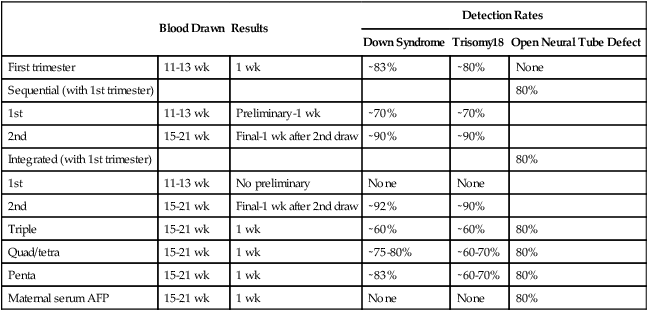
Blood Drawn
Results
Detection Rates
Down Syndrome
Trisomy18
Open Neural Tube Defect
First trimester
11-13 wk
1 wk
∼83%
∼80%
None
Sequential (with 1st trimester)
80%
1st
11-13 wk
Preliminary-1 wk
∼70%
∼70%
2nd
15-21 wk
Final-1 wk after 2nd draw
∼90%
∼90%
Integrated (with 1st trimester)
80%
1st
11-13 wk
No preliminary
None
None
2nd
15-21 wk
Final-1 wk after 2nd draw
∼92%
∼90%
Triple
15-21 wk
1 wk
∼60%
∼60%
80%
Quad/tetra
15-21 wk
1 wk
∼75-80%
∼60-70%
80%
Penta
15-21 wk
1 wk
∼83%
∼60-70%
80%
Maternal serum AFP
15-21 wk
1 wk
None
None
80%
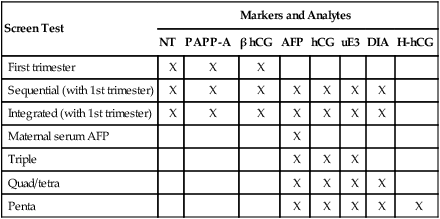
Screen Test
Markers and Analytes
NT
PAPP-A
β hCG
AFP
hCG
uE3
DIA
H-hCG
First trimester
X
X
X
Sequential (with 1st trimester)
X
X
X
X
X
X
X
Integrated (with 1st trimester)
X
X
X
X
X
X
X
Maternal serum AFP
X
Triple
X
X
X
Quad/tetra
X
X
X
X
Penta
X
X
X
X
X
Maternal Serum Alpha Fetoprotein
Triple Screen
Quadruple Screen
Penta Screen
Invasive Diagnostic and Genetic Testing
Chorionic Villus Sampling
Amniocentesis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Genetic Testing