Yasuaki Arai
Ardis first described the synthesis of cyanoacrylates in 1949,1 and since that time, its use for applications such as wound closure, skin grafts, and organ anastomoses has been investigated.2,3 N-butyl cyanoacrylate (NBCA) has been used since the 1980s as a liquid embolic material, mainly for neurointerventional indications; it was approved in the United States by the U.S. Food and Drug Administration for use in cerebral arteriovenous malformations in 2000. Since that time, its use in the periphery has grown significantly. Presently, glue is one of the most important and indispensable embolic materials in interventional radiology. NBCA is approved as a medical device in most countries, but its approval for embolization varies from country to country. Therefore, it is often used off-label for embolization.
DEVICE DESCRIPTION
NBCA is a liquid embolic agent that consists of a two-carbon ethylene molecule with a cyano group and an ester (carbonyl group) attached to one of the carbons; the ester in NBCA is attached to an N-butyl hydrocarbon.4,5 Polymerization occurs upon contact with any ionic substances (e.g., blood, saline, ionic contrast media, and vessel endothelium) due to bonding of the ethylene units after exposure to an anion (such as a hydroxyl group).1 Because of this mechanism, NBCA is able to flow through the vasculature to the target lesion as a liquid but leads to embolization once it polymerizes and becomes solid.
Once administered, the polymerization process causes the release of formaldehyde, which can contribute to the toxicity of NBCA. The extent of the toxicity depends on the size of the side chain ester; NBCA is less toxic than methyl and ethyl cyanoacrylates.6 Embolization with NBCA leads to an acute inflammatory response in the vessel wall and surrounding tissue.1 With time, this leads to a chronic granulomatous inflammatory process.7–9
TECHNIQUE
To use NBCA appropriately as an embolic agent in interventional procedures, there are two points to keep in mind: visualization under image guidance and control of the polymerization time. Both of these points can be addressed with the use of iodized oil (Lipiodol), which can be mixed with NBCA at any ratio to make it visible under fluoroscopy and to increase its polymerization time and viscosity (Figs. 9.1 and 9.2).10 The dilution rate is usually 10% to 50% (NBCA/Lipiodol) for most clinical indications.
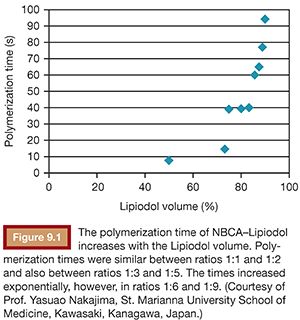
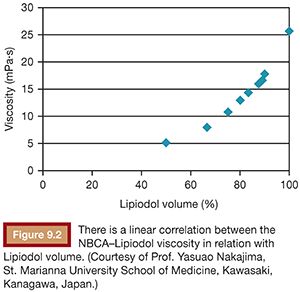
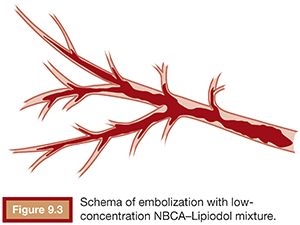
The viscosity of the NBCA–Lipiodol mixture becomes higher upon contact with blood and endothelium in vessels. This ultimately leads to vascular occlusion once blood flow cannot push the mixture due to its high viscosity. This mechanism of embolization is different when compared with materials such as microspheres, polyvinyl alcohol (PVA), and gelatin sponge, which typically occlude vessels at a level within the vasculature, which matches the size of the embolic agent. An NBCA–Lipiodol mixture also stops at the point of branching vessels once the mixture cannot get into smaller vessels due to its high viscosity. Therefore, high-concentration NBCA–Lipiodol mixtures with a dilution rate of 40% to 50% can be used for proximal embolization. Dilution rates of 10% to 20% can be used to embolize long and branching vessels (Fig. 9.3). However, when compared with particles measuring 100 to 500 µm in diameter, an NBCA–Lipiodol mixture leads to a more proximal embolization.
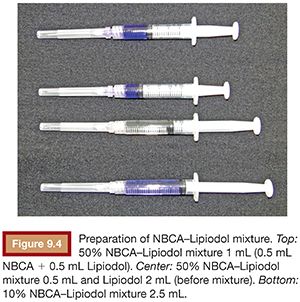
When preparing the mixture of NBCA and Lipiodol, attention is required to avoid any contact between NBCA and Lipiodol and any ionic substance.5 The author always aspirates NBCA from the NBCA ampule directly into a new syringe with a new needle and then aspirates the desired volume of Lipiodol into the same syringe. If a high-diluted NBCA–Lipiodol is to be prepared, a second Lipiodol-containing syringe should be prepared, and both should be mixed to obtain the target dilution rate for the NBCA–Lipiodol mixture (Fig. 9.4). To avoid polymerization, the NBCA–Lipiodol mixture should not be allowed to be in contact with room air for long period. The syringe containing the NBCA–Lipiodol mixture should therefore be capped at all times. The author usually uses the mixture within 10 minutes after preparation.
When injecting the NBCA–Lipiodol mixture, a coaxial catheter system must be used because it is difficult to control the injection with a larger lumen catheter. When the coaxial microcatheter tip reaches the target point, a test injection of contrast is performed to confirm the position of catheter tip and to assess blood flow. The catheter is then flushed with a 5% glucose solution to clear all contrast from the inner lumen.5,11 This is an important step in the use of NBCA because it is difficult to distinguish the NBCA–Lipiodol mixture from contrast under fluoroscopy. Clearing the contrast from the catheter after the test injection allows the operator to confirm when NBCA is being administered. Three seconds after an optimal amount of NBCA–Lipiodol has been injected, the microcatheter should be removed to minimize the risk that the tip of the catheter will adhere to the vessel wall. A small amount of time is needed because if the catheter is removed too quickly, the risk of backflow of unpolymerized NBCA can occur due to the negative pressure caused by catheter removal. If the intent of the procedure is to inject a fixed amount of NBCA–Lipiodol mixture, it is possible to push the mixture with 5% glucose. Following embolization, the microcatheter should be disposed of immediately because the polymerized NBCA–Lipiodol mixture may remain in the lumen of the catheter. It may be possible to flush the catheter with sufficient volume of Lipiodol and 5% glucose if necessary.11 If a guidewire can be passed without any resistance after flushing, the author sometimes uses the same microcatheter system.
CLINICAL APPLICATIONS
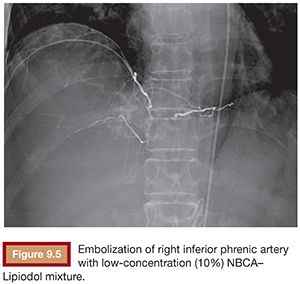
Based on its special features, the use of NBCA as an embolic agent is theoretically indicated for the following three situations. First, it is optimal to occlude a vessel with multiple branches or communications to other vessels. For example, NBCA can be used to embolize atypical vessels responsible for the arterial supply to liver tumors, such as the inferior phrenic artery. The inferior phrenic artery is rather long and has many communications with other arteries (e.g., intercostal arteries, internal mammary artery, etc.). Given the potential territory supplied by this vessel, NBCA is an effective agent to use for embolization in this situation (Fig. 9.5). For this reason, NBCA is a commonly used agent for the treatment of arteriovenous malformations (Fig. 9.6).12 Second, NBCA is used to occlude target vessels when the catheter position is unstable. Catheter stability is very important when using coils for embolization because catheters are under tension when coils are being introduced. If a coil is inserted through a catheter with an unstable position, the catheter tip may move back and forth, which can lead to coil migration into an unexpected or undesired location. Instead of coils, NBCA–Lipiodol mixture can be used in this scenario because it comes out of the catheter in a few seconds. This may often be the case when embolizing a right gastric artery for the purpose of redistribution for hepatic infusion chemotherapy and yttrium 90 infusion (Fig. 9.7),13 portal vein branches for preoperative portal vein embolization (Fig. 9.8),14–16 or a bronchial artery in patients with hemoptysis (Fig. 9.9).17 NBCA has also been shown to be an effective agent to treat gastrointestinal bleeding.18 Third, NBCA–Lipiodol can potentially be a more effective embolic agent than coils or particles in coagulopathic patients.19,20 Coils and particles rely on normal coagulation for vessel occlusion. Therefore, if a patient with bleeding is coagulopathic, coils or particles may not help to stop the bleeding. However, NBCA mixture can mechanically occupy the intravascular lumen and stop blood flow regardless of blood coagulability.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








