and Zdeněk Fryšák1
(1)
Department of Internal Medicine III – Nephrology, Rheumatology and Endocrinology, Faculty of Medicine and Dentistry, Palacky University Olomouc and University Hospital Olomouc, Olomouc, Czech Republic
Keywords
Graves’ diseaseColor flow Doppler ultrasoundPattern III—“thyroid inferno”Peak systolic velocity4.1 Essential Facts
In 1835, Irish physician Robert James Graves published in the London Medical and Surgical Journal a paper titled “Newly observed affection of the thyroid gland in females,” in which he described three women who exhibited “violent and long continued palpitations, [whose] eyeballs were apparently enlarged, [and] a beating of the heart could be heard during the paroxysm at some distance from the bed.” With this publication Graves secured his legacy within the annals of clinical endocrinology [1].
In March 1840, a physician in Merseburg, Germany, Carl Adolph von Basedow, published article in the reputable medical journal of the time, Heilkunde fuer die Gesamte Medizin, titled, in German, “Exophthalmus durch Hypertrophie des Zellgewebes in der Augenhoehle” (“Exophthalmos due to hypertrophy of the cellular tissue in the orbit”). Thereafter, the cardinal symptoms—exophthalmos, goiter, and tachycardia—became known as the “Merseburger triad.” Since 1858 Basedow’s disease has been the most commonly used identifying term for the disease on the European continent, while Graves’ disease (GD) is more widely used in the English-speaking world [2].
Graves’ disease is the most common cause of hyperthyroidism, followed by toxic multinodular goiter. Rarer causes include an autonomously functioning thyroid adenoma and thyroiditis [3].
Prevalence of hyperthyroidism in women is between 0.5 and 2%, and is 10 times more common in women than in men in iodine-replete communities [3].
Prevalence data in elderly persons shows a wide range between 0.4 and 2.0% [3].
Incidence data available for overt hyperthyroidism in men and women from large population studies are comparable, at 0.4 per 1000 women and 0.1 per 1000 men, but the age-specific incidence varies considerably [3].
GD has the highest incidence in between 20 and 49 years of age, with a secondary peak at the age of 60–69 years. In contrast, the peak age-specific incidence of hyperthyroidism caused by toxic nodular goiter and autonomously functioning thyroid adenomas is >80 years [3].
Hyperthyroidism is rare in childhood and is most commonly caused by GD. It affects 0.02% of children, or 1 in 5000. The peak incidence occurs from 11 to 15 years of age with a female predominance. A positive family history is common [4].
GD as a thyroid autoimmunity is caused by stimulatory autoantibodies against the thyrotropin receptor (TSHR-Ab). These antibodies cause uncontrolled and continuous stimulation of the thyroid, leading to excessive synthesis of the thyroid hormones: thyroxine (T4) and triiodothyronine (T3) and its hypertrophy.
Laboratory findings: Measurements of serum TSH and free thyroxine (fT4) levels are essential for evaluation of hyperthyroidism, and TSHR-Ab shows high specificity of 99% and a sensitivity of 95% for confirmation of GD diagnosis [5].
Clinical findings: Symptoms of hyperthyroidism and specific features which may point to GD include the presence of a diffuse goiter, ophthalmopathy and pretibial myxedema, and acropachia. Patients with GD usually have diffuse, nontender, symmetrical enlargement of the thyroid gland. Ophthalmopathy, consisting of protrusion of the eyes with periorbital soft-tissue swelling and inflammation, and inflammatory changes in the extraocular muscles resulting in diplopia and muscle imbalance, is clinically evident in 30% of patients with GD [6, 7].
Hyperthyroidism occurs in approximately 0.2–1.0% of all pregnancies. Gestational transient thyrotoxicosis (GTT) is the most common cause as a result of the thyroid stimulatory actions of human chorionic gonadotropin (hCG). However, for management it is important to distinguish GD found during pregnancy to GTT (no past history or family history of thyroid autoimmunity, no goiter, no ophthalmopathy, TSHR-Ab negative, TPO-Ab negative, may present with hyperemesis, dehydration and electrolyte imbalance, is self-limiting and usually does not require antithyroid drugs treatment) [6].
Radioiodine 131I–therapy (RIT) has been used to treat hyperthyroidism since the 1940s. Generally, after 4–6 weeks thyroid function normalizes [5].
4.2 US Features of Graves’ Disease
US imaging of classical GD pattern [4, 12]:
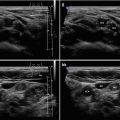
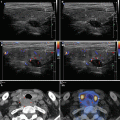
A diffusely heterogeneous or coarse echotexture (Fig. 4.1aa).
A diffusely hypoechoic (Fig. 4.1aa).
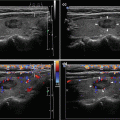
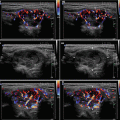
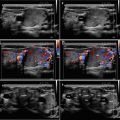
CFDS demonstrate markedly increased vascularity pattern III—“thyroid inferno” (Figs. 4.1bb and 4.4bb).
CFDS parameter peak systolic velocity (PSV) of inferior thyroid artery (ITA) with cut-off value of 65 cm/s, mostly over 150 cm/s (Fig. 4.1ii).



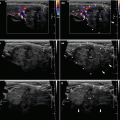
Fig. 4.1
(aa) A 33-year-old woman with Graves’ disease (GD) and large goiter volume 65 mL. US overall view: diffusely enlarged thyroid gland with round-shaped lobes; inhomogeneous structure; mixed echogenicity; ventrally mostly hypoechoic micronodular structure; hyperechoic transverse septum at dorsal part of RL; Tvol 65 mL, asymmetry—RL 42 mL and LL 23 mL; transverse, depth of penetration 4.5 cm. (bb) Overall view of GD, CFDS: diffuse hypervascularity, pattern III—“thyroid inferno”; transverse. (cc) Detail of RL with GD: coarsened structure; mixed echogenicity; ventrally mostly hypoechoic micronodular structure with short fibrous septa; longitudinal. (dd) Detail of RL with GD, CFDS: diffuse hypervascularity, pattern III—“thyroid inferno”; longitudinal. (ee) Detail of LL with GD: inhomogeneous structure; mostly isoechoic, ventrally hypoechoic micronodular structure; longitudinal. (ff) Detail of LL with GD, CFDS: diffuse hypervascularity, pattern III—“thyroid inferno”; longitudinal. (gg) Detail of GD, left inferior thyroid artery (ITA) with two branches: small lumens located between low pole of LL and left CCA; transverse. (hh) Detail of GD, CFDS in the left ITA: small lumens located between low pole of LL and left CCA; transverse. (ii) Detail of GD, flow parameter measured in the left ITA: peak systolic velocity (PSV) 147 cm/s, confirming diagnosis of GD
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree