Gynecologic Concepts
Female patients with acute lower abdominal or pelvic pain often represent a diagnostic challenge. The differential diagnosis is broad (Table 16-1), and the workup often requires multiple diagnostic tests. Ultrasound is the initial diagnostic imaging modality of choice in the majority of cases. Point-of-care ultrasound, performed and interpreted by the clinician, and completed at the time of the initial physical examination, helps narrow the differential diagnosis and may eliminate the need for further diagnostic testing.
 TABLE 16-1. DIFFERENTIAL DIAGNOSIS OF LOWER ABDOMINAL PAIN IN FEMALE PATIENTS
TABLE 16-1. DIFFERENTIAL DIAGNOSIS OF LOWER ABDOMINAL PAIN IN FEMALE PATIENTS
GI
Appendicitis
Inflammatory bowel disease
Irritable bowel syndrome
Constipation
Gastroenteritis
Diverticulitis
URINARY TRACT
Cystitis
Pyelonephritis
Nephrolithiasis
REPRODUCTIVE
Ectopic pregnancy
Intrauterine pregnancy
Pelvic inflammatory disease
Tubo-ovarian abscess
Ovarian cyst
Hemorrhagic functional cysts
Ovarian torsion
Mittelschmerz
Dysmenorrhea
Endometriosis
 CLINICAL CONSIDERATIONS
CLINICAL CONSIDERATIONS
Imaging the pelvis is a crucial step in the evaluation of women with lower abdominal pain or pelvic pain. Accurate management is predicated on choosing the most effective diagnostic tool. Four diagnostic modalities are available for evaluating the pelvis: ultrasound, CT, MRI, and laparoscopy.
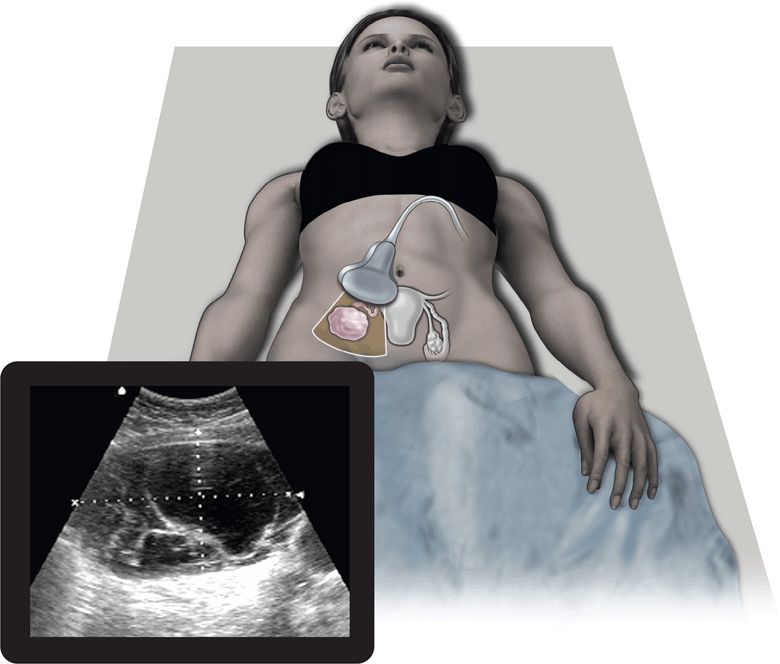
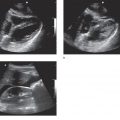
Ultrasound has proven to be a rapid, noninvasive, portable, repeatable, inexpensive, and accurate method for visualizing and diagnosing pathology within the pelvis. Several advantages over CT, MRI, and even the bimanual pelvic examination have made ultrasound the first-line diagnostic imaging modality in patients with acute pelvic pain or masses.1–3 Both transabdominal and endovaginal ultrasound can be used by the clinician at the bedside during the initial physical examination. The use of point-of-care ultrasound has far-reaching benefits to patient care. It may identify specific diseases in the differential diagnosis and often eliminates the need for expensive and time-consuming diagnostic tests (Figure 16-1). In addition, ultrasound does not expose patients to ionizing radiation. Since the clinician performs the point-of-care ultrasound examination, patients perceive this as more time spent with their physician. This serves to improve patient satisfaction, provides them with more time to ask questions, and ultimately increases their confidence in the physician and their understanding of the diagnosis. Another advantage unique to ultrasound is the ability of color flow Doppler to evaluate pelvic organs for adequacy of blood flow.
Figure 16-1. The top image refers to the transverse placement of a large footprint curved array transducer. The bottom image depicts a complex ovarian mass, one of the many entities that lies within the differential diagnosis for women presenting with acute pelvic pain.
The main disadvantage of ultrasound with respect to the other imaging modalities is its limited scope. Other imaging modalities, such as CT and MRI, may yield valuable information about other organ system pathology and the extent to which a disease process may have progressed. Also, sonograms are sometimes technically inadequate due to interference from bowel gas.
While CT is used routinely for the preoperative evaluation of masses that are suspicious for malignancy, it is generally considered a second-line imaging modality to ultrasound for the evaluation of pelvic pain. The advantage of CT is its ability to image the full extent of a large adnexal lesion that cannot be visualized in its entirety with sonography alone. Another advantage of CT is its usefulness in diagnosing GI sources of pain, such as appendicitis and diverticulitis. The major disadvantages to CT are radiation exposure and cost. CT emits ionizing radiation.4
Although MRI is also considered a second-line imaging modality, it has several advantages over CT and ultrasound. MRI does not expose the patient to radiation and provides more detailed information for the detection of subtle tissue differentiation of pelvic organs. MRI has better tissue resolution than ultrasound, and is therefore more accurate in diagnosing pelvic inflammatory disease (PID) and pelvic masses. A 1999 study compared MRI with endovaginal ultrasound for the diagnosis of laparoscopy-proven PID. Of the 21 patients proven to have PID, MRI diagnosed 20 (95%) patients while endovaginal ultrasound correctly diagnosed 17 (81%) patients.5 Many of the same disadvantages of CT—cost, availability, lack of portability—apply to MRI as well.
While laparoscopy remains the gold standard for the diagnosis of PID and pelvic masses, its use may not be readily available or justified in screening patients with vague symptoms. It is invasive, costly, time-consuming, results in scarring, and requires the small but real risk of general anesthesia. The advantage of laparoscopy, however, is the ability to reveal other pathologic conditions that have been misdiagnosed as PID. In one study, 12% of patients diagnosed with PID revealed other pathologic findings during laparoscopy, such as appendicitis or endometriosis.6 Another advantage of laparoscopy is the ability to intraoperatively intervene in a pathologic process, such as the untwisting or resection of a torsed ovary, drainage of an abscess, or appendectomy.
 CLINICAL INDICATIONS
CLINICAL INDICATIONS
Clinical indications for performing pelvic ultrasound include:
 Acute pelvic pain
Acute pelvic pain
 Acute pelvic inflammatory disease
Acute pelvic inflammatory disease
 Evaluation of pelvic or adnexal masses
Evaluation of pelvic or adnexal masses
ACUTE PELVIC PAIN
Acute pelvic pain in women is a common complaint in the emergency or ambulatory care setting. The differential diagnosis is vast. Although life-threatening conditions such as ectopic pregnancy are in the differential, the majority of patients can be treated and discharged home. While the definitive evaluation of these patients ultimately may involve CT, pelvic sonography is the diagnostic imaging modality indicated in their initial evaluation.
Ovarian Torsion
This entity should be considered in the differential diagnosis of any woman with lower abdominal pain (Table 16-2). Ovarian torsion is a GYN emergency that can result in both reproductive and hormonal compromise if not promptly diagnosed and treated. Because the diagnosis is often elusive and sufficiently delayed, detorsion of the ovary is rarely an option. A twisting of the ovarian attachments through the utero-ovarian ligament to the uterus and through the infundibulopelvic ligament to the pelvic sidewall results in congestion of the ovarian parenchyma and eventual hemorrhagic infarction from decreased ovarian blood supply.7 The “classic” symptoms of acute, severe, unilateral lower abdominal or Appendicitis Adnexal mass Pelvic mass Myoma Ectopic pregnancy Tubo-ovarian abscess Ruptured viscus pelvic pain are present only in approximately one-third of the patients with confirmed ovarian torsion. Ovarian torsion is frequently missed on the preoperative diagnosis; the two most common incorrect preoperative diagnoses are tubo-ovarian abscess (TOA) and ruptured corpus luteum cyst.8
 TABLE 16-2. DIFFERENTIAL DIAGNOSIS OF OVARIAN TORSION
TABLE 16-2. DIFFERENTIAL DIAGNOSIS OF OVARIAN TORSION
Appendicitis
Adnexal mass
Pelvic mass
Myoma
Ectopic pregnancy
Tubo-ovarian abscess
Ruptured viscus
Torsion can occur in normal ovaries, but this would be an unusual occurrence. In general, for torsion to take place, the ovary or tube must be enlarged such as in the case of a mass or cyst as part of or immediately adjacent to the ovary or fallopian tube. These masses are thought to act as a fulcrum by which torsion can propagate. One study demonstrated that ovarian torsion was associated with palpable adnexal masses in over 90% of adults compared with only 50% of children.9 Others reported that a unilaterally enlarged ovary with small peripherally located cysts (1–6 mm) was the most common finding (56%) in young and adolescent girls.10 In 1985, it was reported that ovarian masses were associated with cases of torsion in only 50% of patients. Pregnancy appears to be a risk factor as well; 20% of all cases can be found to occur during pregnancy.8
Abnormal blood flow detected by Doppler sonography is highly predictive of ovarian torsion and is therefore useful in the diagnosis of ovarian torsion. Failure to identify arterial waveforms is highly suggestive of ovarian torsion. When normal flow is detected by Doppler sonography, it does not necessarily exclude ovarian torsion. In fact, ovarian torsion is missed in 60% of these cases, and time to diagnosis is therefore delayed. In patients undergoing hormonal therapy for ovarian stimulation, the sensitivity of Doppler for ovarian torsion increases to 75%.11 Despite being intuitively similar to other organs, such as the testicle, lack of blood supply to the ovary cannot be adequately excluded using Doppler. The reason for this is twofold. First, Doppler flow may be present in one part of the ovary (peripheral or central) but not in the other because the ovary has a dual blood supply. Second, thrombosis of venous structures produces the symptoms of ovarian torsion prior to the arterial system becoming occluded. While some authors have suggested that absent blood flow on spectral Doppler and color Doppler is specific for torsion,12,13 others suggest that observing blood flow to the ovary should not be relied upon to definitively exclude this diagnosis.14 One study reported that the presence of a Doppler signal was present in 9 of 14 patients ultimately proven to have ovarian torsion.10
Gray scale findings may be useful in diagnosing ovarian torsion by identifying a large ovary with enlarged follicles or an enlarged complex cystic adnexal mass. Conversely, it has been suggested that normal ovarian size and texture may be helpful in excluding this diagnosis. One study evaluated 41 patients suspected of having ovarian torsion who had undergone transabdominal ultrasound. Of the 11 patients who had ovarian torsion proven at surgery, 7 were correctly diagnosed by ultrasound. Ovarian enlargement was detected in all 11 patients. This study (albeit a very small sample size) yielded a positive predictive value of 87.5%. In the other 28 patients, sonography correctly excluded the diagnosis, yielding a specificity of 93%. All patients were followed for 63 months on an outpatient basis.7
Vaginal Conditions
Some GYN procedures involve instrumentation that result in postoperative complications. These patients may present with vaginal bleeding, acute pelvic pain, and unstable vital signs. Ultrasound can play a crucial role in the timely diagnosis and management of these patients. For example, ultrasound can localize and diagnose a vaginal hematoma in a hypotensive patient who recently underwent a dilatation and curettage procedure. Typically, the ultrasound examination is performed transabdominally in an attempt to localize the hematoma within the vaginal tissue planes.
ACUTE PELVIC INFLAMMATORY DISEASE
Acute PID, defined as an infection in the upper genital tract, represents a spectrum of disease entities, including any combination of endometritis, salpingitis, oophoritis, pelvic peritonitis, and TOA.15 More than 1 million women are diagnosed with PID annually and 25% of them proceed to suffer at least one sequela of PID, which include infertility, ectopic pregnancy, or chronic abdominal pain.16 The severity of clinical presentation corresponds poorly with the damage to the fallopian tubes. Many young women with PID have mild and vague symptoms.17 Therefore, the diagnosis of PID on clinical grounds has been notoriously difficult and was shown to be only 66% accurate in one study.18 It is not surprising that endovaginal ultrasound was demonstrated to be superior to bimanual examination alone in the diagnosis of findings consistent with PID.19
Early sonographic signs of PID are increased adnexal volume and periovarian inflammation with fluid collections. On ultrasound, this appears as structures that lack the distinct margins that are normally identified. Another sonographic sign of PID is the decreased ability of the ovary to slide smoothly in the adnexa (sliding organ sign) when the ultrasound transducer is inserted and withdrawn from the vagina. This sign suggests that the ovary has been tethered to the fallopian tube by inflammatory adhesions. These sonographic findings were correlated with laparoscopic evidence of periovarian exudates and adhesions.20 In 1992, it was demonstrated that sonographic evidence of free fluid had a sensitivity of 77% and specificity of 79% in culture-proven PID. Finally, the presence of “polycystic-like” ovaries containing increased stroma with several follicles scattered throughout the stroma has been found to be indicative of PID; one study demonstrated a sensitivity of 100% and a specificity of 71% for this finding.21
Another study evaluated four ultrasound markers to suggest evidence of PID: free fluid in the cul-desac, multicystic ovaries, visualization of fallopian tube or tubal fluid, and presence of an adnexal mass or TOA. This study found that in patient populations that have a high prevalence of PID, an endovaginal ultrasound examination positive for these markers is useful for suggesting the diagnosis of PID, and thus helping avoid laparoscopy. A negative ultrasound examination, however, should not be viewed by the clinician as being reliable for excluding the diagnosis of PID in a patient who appears clinically ill. In this subset of patients, laparoscopy may be required to make the diagnosis.22
EVALUATION OF PELVIC OR ADNEXAL MASSES
Hydrosalpinx
Since hydrosalpinx is present only in abnormal conditions such as PID, TOA, or ectopic pregnancy, its finding should immediately raise a red flag. Fluid in the fallopian tube can be encountered after tubal ligation.
Tubo-Ovarian Abscess
Women who present with a pelvic mass may have a TOA, tubo-ovarian complex, uterine fibroid, hydrosalpinx, ovarian cyst, or a variety of other complex adnexal masses. Clinicians cannot rely solely on their bimanual examination to accurately detect pelvic masses; 70% of pelvic masses found on ultrasound examination were initially missed during the bimanual examination.23 A pelvic mass detected on physical examination is an indication for a pelvic ultrasound examination. If a cystic structure is found within the ovary, this may provide a finding for the clinician to explain the patient’s symptoms and a clear disposition that often negates further workup during that visit. The presence of a cystic structure on the ovary, however, is very common and does not exclude the presence of other concomitant pathology. Furthermore, if the cyst is large enough, typically over 3.5 cm, ovarian torsion may need to be considered.
Pelvic ultrasonography is indicated in cases of severe, recurrent PID with or without the presence of a mass on physical examination. It is critically important that a distinction be made between PID and TOA to direct specific treatment regimens. Since the same clinical diagnostic difficulties of PID apply to TOA, ultrasound plays a crucial role in the diagnosis. Understanding that the development of a TOA occurs through a stepwise fashion will aid the clinician with the ultrasound examination. The first stage involves inflammation of the tubal mucosa. The wall eventually thickens and purulent material fills the lumen and spills into the cul-de-sac. If either end of the fallopian tube becomes blocked, a pyosalpinx can occur. As the pressure within the lumen increases, the walls are stretched thin and the tube becomes distended. In some patients, the process stops at this stage, resulting in chronic hydrosalpinx. When the remnants of the endosalpingeal folds become fibrotic, they appear as spokes outlined by anechoic fluid (“cogwheel sign”). When interrogated using power Doppler, marked hyperemia is seen throughout this complex structure. As the acute inflammatory process continues to proceed, it erodes through the distended wall. If the ovary has a recent defect from the ruptured corpus luteum, it becomes exposed to this inflammation and purulent material enters this space. The final stage of abscess formation occurs when the pus walls itself off, fusing the tube and ovary together.
The incidence of PID developing into TOA has been reported to be between 4%24 and 30%.25 TOA requires a different treatment regimen than PID since it forms an abscess, tends to be polymicrobial, and consists of anaerobes.26 Since the mid-1970s, ultrasonography has been shown to be an accurate, sensitive, and noninvasive imaging technique for diagnosing TOA.27,28 Furthermore, serial ultrasound examinations have proven to be useful in following a TOA that is managed nonoperatively. Pelvic ultrasound also assists in the selection of the most effective treatment regimen.26,29 In a study of 106 patients with clinically suspected PID, ultrasound findings demonstrated 19 patients with pyosalpinx and 4 patients with hydrosalpinx. These 23 patients had their medical therapy directly altered as a result of the endovaginal ultrasound.30
Uterine Fibroids
Uterine fibroids represent the most common GYN tumor. Leiomyomas start as a mass of smooth muscle proliferation in a whorled spherical configuration. Atrophy and vascular compromise eventually ensue, which result in necrosis and calcification. These patients can present with pelvic pain, dysuria, dysmenorrhea, constipation, or low back pain (from compression of lumbar plexus).
 ANATOMICAL CONSIDERATIONS
ANATOMICAL CONSIDERATIONS
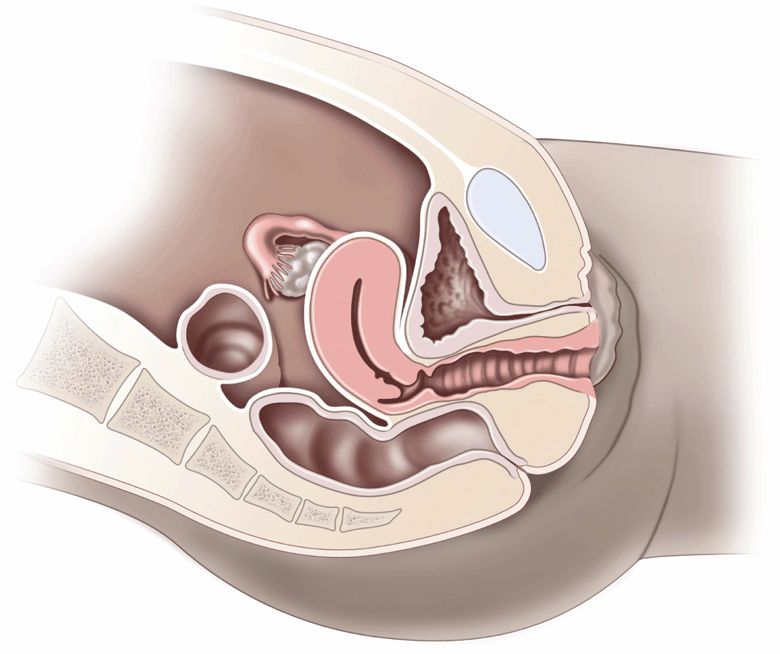
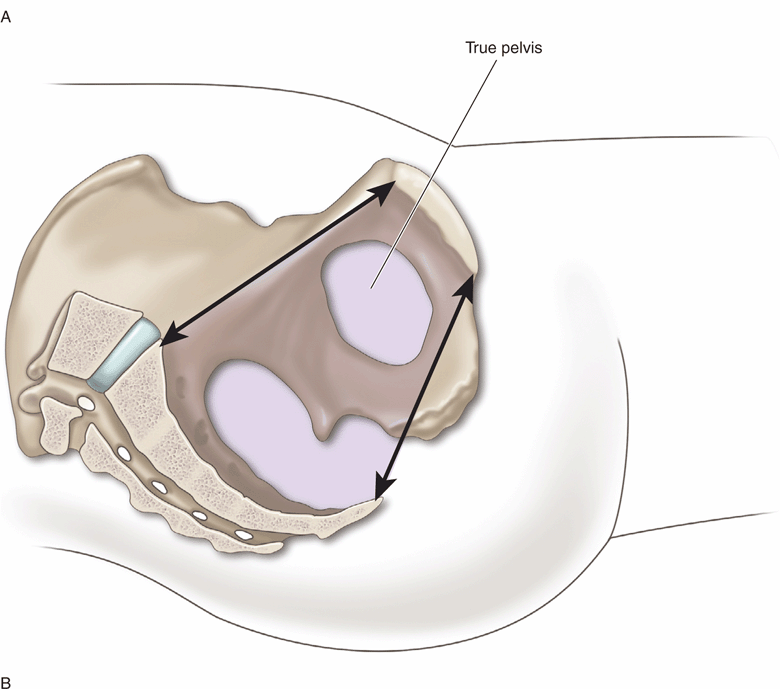
To understand the pelvic anatomy, it may be helpful to think of the pelvis as two distinct regions: the true pelvis and false pelvis. The true pelvis has a basin-shaped contour and is bounded anteriorly by the pubic symphysis and pubic rami. It is bounded posteriorly by the sacrum and coccyx and inferiorly by the perineal musculature. The false pelvis is located superior to the true pelvis. The abdominal wall represents its anterior border, the iliac bones define its lateral border, and the sacral promontory outlines its posterior border. The empty bladder lies within the true pelvis and, when distended, enters the false pelvis (Figure 16-2A and B).
The uterus is a thick-walled, muscular structure whose shape can vary with cyclical menstrual changes and distention of the bladder. Typically, the uterus is found in the anteverted position in its relationship with the bladder; in 25% of women, it is retroflexed. During the reproductive years, the uterus measures up to 7 cm × 4 cm × 5 cm. The postmenopausal uterus measures 7 cm in length and 1–2 cm in transverse. The endometrial thickness varies with the menstrual cycle from 6 mm to less than 1 mm following menstruation.
The ovaries are elliptical-shaped structures and are found in a range of positions in the parous woman. In the nulliparous woman, the ovaries are typically located on the posterolateral wall of the true pelvis, adjacent to the internal iliac vessels. The menstrual cycle is categorized into two phases: the proliferative phase, which culminates in ovulation, followed by the secretory phase, which ends in menstruation. Cystic follicles regularly occur during the proliferative phase and are not technically termed a “cyst” until they reach a diameter of 2.5 cm. A corpus luteum then forms at the site of ovulation during the secretory phase, but rarely lasts for more than 6 weeks in the nonpregnant patient. Therefore, in the absence of ovulation, these cysts cannot occur. Once ruptured, the only evidence of their existence may be the presence of free fluid in the posterior cul-de-sac or near the ovary.31
The pouch of Douglas is a term that refers to the potential space in the posterior cul-de-sac of the pelvis. It consists of the peritoneal reflection posterior to the uterus and anterior to the rectosigmoid colon. Because this is the most dependent portion of the supine woman, a trace of free fluid is normally seen here, especially in the 5 days prior to menstruation.32 The anterior cul-desac lies between the bladder (anterior) and the uterus (posterior). Since this potential space is not dependent, it only contains free fluid when a significant amount is present in the pelvis.
 GETTING STARTED
GETTING STARTED
Foremost in importance is patient positioning. Failure to allow for a full range of motion of the transducer handle will often result in inadequate imaging. Allowing the buttocks to come to the edge of a lithotomy table while the feet are securely placed in stirrups is the ideal position for endovaginal scanning. In lieu of an available GYN gurney, one can elevate the patient’s hips by placing several towels under the buttocks. This will allow for full transducer handle movement when attempting to visualize anterior structures. The second most important factor in endovaginal ultrasound is instructing the patient to empty her bladder. Even small amounts of urinary volume can move the uterus and ovaries away from the tip of the endovaginal transducer and significantly alter image quality. Conversely, scanning via the transabdominal route is facilitated by urinary bladder volume.
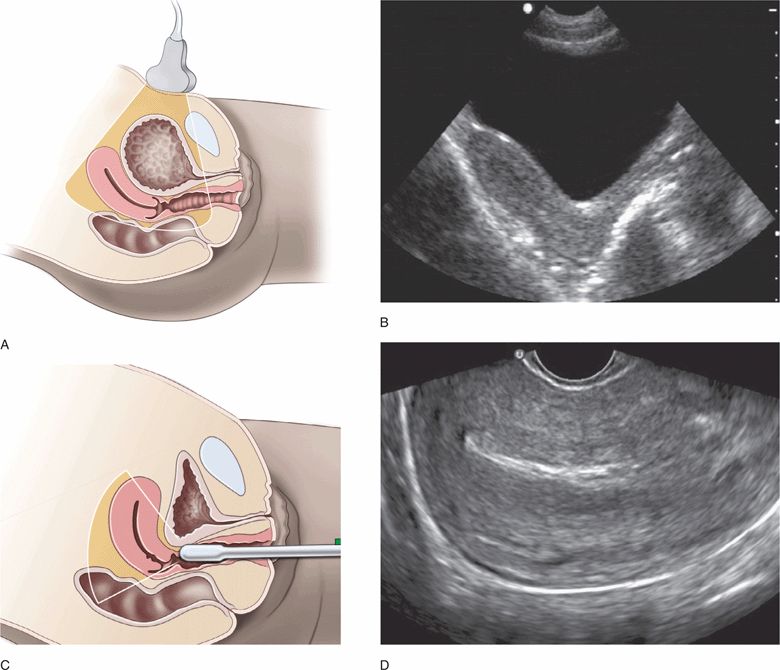
Endovaginal pelvic ultrasound may initially be confusing, but it is actually more straightforward than trans-abdominal pelvic ultrasound. It is important to learn the standard transducer orientations and understand the very different views used for transabdominal versus endovaginal imaging. Transabdominal views are usually obtained with a full bladder (Figure 16-3A,B), and endovaginal views are usually obtained with an empty bladder (Figure 16-3C,D). It is crucial to understand that an anteverted uterus changes position significantly depending on the volume of urine in the bladder. This explains how the tip of the transducer can touch the lateral wall of the uterus during endovaginal scanning despite the fact that the vaginal stripe and the body of the uterus are not adjacent structures in transabdominal images.
Figure 16-3. (A) Position of the uterus and imaging sector when the bladder is full for transabdominal ultrasound and (C) empty for endovaginal ultrasound. Comparison of ultrasound resolution of the uterus in the same patient using transabdominal (B) and endovaginal (D) longitudinal views.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 CLINICAL CONSIDERATIONS
CLINICAL CONSIDERATIONS CLINICAL INDICATIONS
CLINICAL INDICATIONS ANATOMICAL CONSIDERATIONS
ANATOMICAL CONSIDERATIONS GETTING STARTED
GETTING STARTED TECHNIQUE AND NORMAL ULTRASOUND FINDINGS
TECHNIQUE AND NORMAL ULTRASOUND FINDINGS COMMON AND EMERGENT ABNORMALITIES
COMMON AND EMERGENT ABNORMALITIES COMMON VARIANTS AND SELECTED ABNORMALITIES
COMMON VARIANTS AND SELECTED ABNORMALITIES PITFALLS
PITFALLS CASE STUDIES
CASE STUDIES