Theresa M. Caridi • Richard Shlansky-Goldberg
BACKGROUND
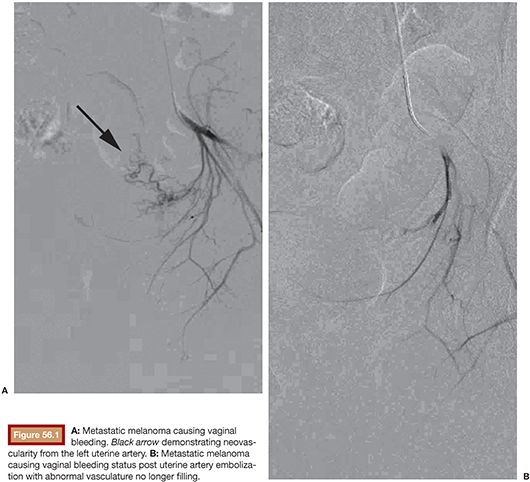
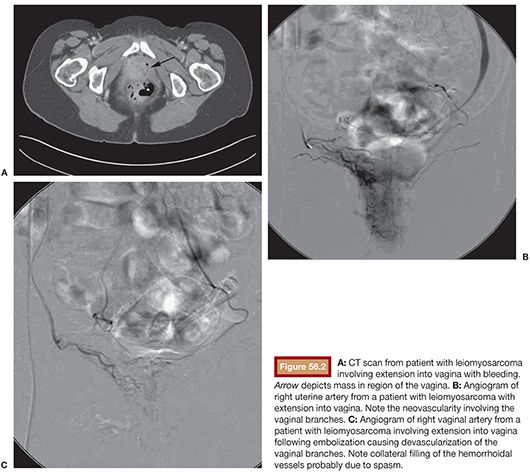
In the United States in 2010, 83,745 womesn were diagnosed and 28,770 died from a gynecologic cancer.1 These include cervical, ovarian, uterine, vaginal, and vulvar cancers and the rare fallopian tube malignancy. In addition, these structures may be involved in metastatic disease from nongynecologic sources. Survival rates for nearly all cancers have improved significantly since the 1970s due largely to earlier detection and/or advances in treatment. Many gynecologic cancers are curable, and the mainstay of treatment remains a combination of surgery, radiation therapy, and chemotherapy. Staging and pathology ultimately determine the treatment type and whether individual or combination therapy is needed. In rare circumstances, embolization of gynecologic malignancies may be employed. These include the setting of life-threatening hemorrhage, preoperatively when there is a predisposition for massive bleeding, and rarely as a treatment of the cancer itself. Treatment of nonhemorrhagic gynecologic malignancy by endovascular methods is infrequent, not only because cure is often achieved by other methods but also because prior treatment with pelvic irradiation increases the risk for uterine necrosis after embolization. Regarding significant bleeding from primary or metastatic disease or as a complication of surgical or radiation therapy, endovascular treatment decreases morbidity and mortality as it is minimally invasive and generally more effective at rapidly achieving cessation of bleeding than surgery (Figs. 56.1 and 56.2).2
Cervical Cancer
The American Cancer Society estimates approximately 12,340 new cases and 4,030 deaths from invasive cervical cancer in 2013.3 Historically, cervical cancer was once one of the most common causes of cancer death for women; however, between 1955 and 1992, the cervical cancer death rate declined by almost 70% owing to the increased use of the Pap test. Cervical cancer is now highly preventable in most Western countries because of both Pap screening and more recently availability of a vaccine to prevent human papillomavirus infections. When found early, cervical cancer is highly treatable and associated with long survival and good quality of life. Treatment usually consists of surgery, radiation therapy, and chemotherapy. The role of embolization is limited due to early detection and curable disease, but it can be useful in the setting of advanced disease with chemoinfusion/embolization, hemorrhage, and also bleeding from a cone biopsy.
Ovarian Cancer
Ovarian cancer accounts for about 3% of cancers among women, but it causes more deaths than any other cancer of the female reproductive system.1 The American Cancer Society estimates approximately 22,240 new cases and 14,230 deaths from ovarian cancer in 2013.3 A woman’s risk of getting ovarian cancer during her lifetime is approximately 1 in 72, and her lifetime chance of dying from ovarian cancer is about 1 in 100, not including low malignant potential ovarian tumors. However, the rate at which women are diagnosed with ovarian cancer has been slowly falling over the past 20 years. The primary treatments for ovarian cancer include surgery, chemotherapy, hormone therapy, targeted therapy (such as bevacizumab), and radiation therapy. Often, a combination of treatments is enlisted. The role for embolization is limited, as combination therapy is often curative, or at least aimed at cure. Additionally, ovarian cancer historically has a low propensity to result in life-threatening hemorrhage.
Uterine Malignancy
The American Cancer Society estimates approximately 49,560 new cases and 8,190 deaths from cancer of the uterus in 2013.3 The death rate for uterine cancer, which was the leading cause of cancer death in the early 20th century, declined from 1930 to 1997 but has since been nearly stable. The grouping of uterine cancer includes both endometrial cancers and uterine sarcomas, with only 2% being uterine sarcoma. The four established methods of treatment for women with endometrial cancer are surgery, radiation therapy, hormonal therapy, and chemotherapy. Again, there is a limited role for embolization except in the setting of hemorrhage. Similarly, a malignancy involving but not originating from the uterus is gestational trophoblastic disease. Like those neoplasms noted earlier, these lesions may also cause uncontrollable bleeding.4
Vaginal and Vulvar Cancer
In 2010, there were 1,211 women in the United States diagnosed with vaginal cancer and 4,305 women with vulvar cancer.1 Vaginal and vulvar cancers are rare, accounting together for approximately 6% to 7% of all gynecologic cancers diagnosed in the United States. Deaths are also low in number, with 423 women in the United States in 2010 who died from vaginal cancer and 942 who died from vulvar cancer. Invasive vaginal and vulvar cancers are treated mainly with radiation therapy and surgery. Chemotherapy in combination with radiation may be used to treat advanced disease. As in other gynecologic malignancies aforementioned, embolization is not a mainstay of treatment apart from the setting of hemorrhage.
In the 1970s, early reports of transarterial embolization for gynecologic malignancy were published. Schwartz et al. reported seven patients, two of whom were bleeding from their malignancy itself and five from bleeding related to therapy.5,6 Four out of the seven patients had bleeding controlled with autologous clot embolization or balloon tamponade, whereas three failed who had bleeding treated with vasopressin. Following this, Athanasoulis et al.7 published the use of gelatin sponge to successfully treat bleeding from radiation used as therapy for two cases of cervical carcinoma. Miller et al.8 also published two cases with gelatin sponge to treat radiation-induced bleeding associated with gynecologic malignancies using a femoral approach in one and a brachial in another. Kelemen et al.9 along with Smith and Wyatt10 demonstrated the technique of bilateral anterior division internal iliac embolization with gelatin sponge for massive vaginal bleeding from pelvic malignancies. In the 1980s, embolization was described as a technique to control bleeding from gestational trophoblastic disease and as a preoperative technique to reduce intraoperative bleeding.11,12
More recently, with the use of microcatheters and permanent embolics such as coils and particles, embolization has become easier and safer; chemotherapy can be added to embolization procedures as well. Arterial infusion with or without embolics is being used to treat ovarian and cervical cancer. In the 1990s, embolization with intra-arterial infusion of chemotherapeutic agents was described.13 Twenty-two patients with advanced gynecologic cancer underwent embolization and infusion of cisplatin with a 73% response rate, allowing a large portion of patients to be surgically resected.
More recent reports with larger numbers of patients with advanced gynecologic cancers have been published, demonstrating the advantage of embolization over surgery in palliative care of patients with bleeding complications from their malignancy.2 Advances in the care of patients with cervical cancer with transuterine arterial chemotherapy have allowed for fertility-sparing management using arterial cisplatin and intravenous nedaplatin or irinotecan and vaginal trachelectomy.14 In addition, a phase II study of 22 patients using transuterine embolotherapy with cisplatin and Gelfoam sponges with intravenous chemotherapy demonstrated 5-year progression-free and overall survival rates in stages IB2 to IIB to be 70.0% and 69.5%, respectively. The 2 patients with stage IVA tumors were alive without recurrence for 72 and 84 months after enrollment.15
TECHNIQUE
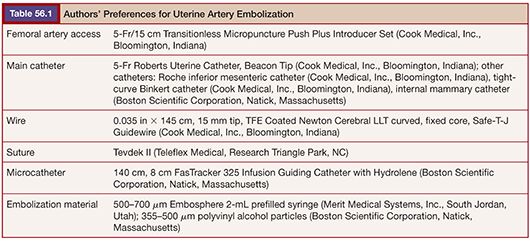
The most likely use of embolization for gynecologic malignancies is in life-threatening hemorrhage and typically involves embolization of the hypogastric or uterine arteries and, less commonly but occasionally, the ovarian arteries. As with treatment of benign disease, there is variation in operator approach to embolization of the pelvic vessels. All are performed in an angiography suite equipped for digital subtraction angiography or in an operating room with similar equipment. The standard approach is right common femoral artery access followed by aortography with a nonselective flush catheter, particularly in the setting of hemorrhage. The flush catheter is positioned at approximately the L1 vertebral body level to allow for imaging of the ovarian arterial origins in addition to the pelvic flow. Selective and superselective catheterization can be performed with any of several 4-Fr or 5-Fr catheters, such as a Cobra, VS, Simmons, or Roberts Uterine Catheter (RUC) (Table 56.1). Many operators will switch to a microcatheter to select the uterine artery for embolization because it will allow greater flow and reduce the incidence of spasm. Others perform bilateral femoral punctures to reduce procedure and radiation time.16 Regardless of the suspected source of hemorrhage, bilateral angiography as well as embolization is often performed owing to the vast pelvic blood supply in uterine disease.
Ipsilateral catheterization is commonly performed with use of the Waltman loop technique but again can be performed by accessing the contralateral common femoral artery in a bilateral puncture approach. Waltman et al.17 described looping of a catheter back over itself by selecting a suitable visceral vessel and pushing the catheter up into the aorta, with attention given to avoid damage to the visceral vessel of choice. Historically, this technique worked well with braided catheters; however, some operators suggest the newer hydrophilically coated 4-Fr and 5-Fr catheters are more susceptible to kinking while the Waltman loop is being formed. Also, these catheters have the tendency to uncoil while the loop is withdrawn into the ipsilateral iliac artery and risk dissecting a vessel while reforming the reverse shape.
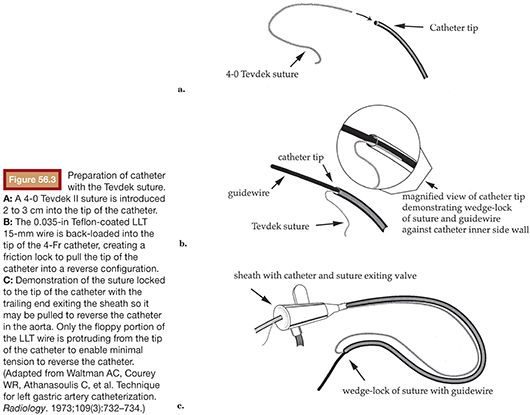
Noting these limitations, Cope18 in 1986 described the catheter reversal maneuver with a suture, which eliminated the need to select a vessel to form a loop, thereby reducing the risk of dissection and atheroemboli. The technique was further refined for uterine artery embolization using a 4-Fr non–tapered angled glide catheter (Boston Scientific Corporation, Natick, Massachusetts) and more recently with the RUC.19 The suture technique involves threading 2 to 3 cm of 4-0 Tevdek II suture into the end of a catheter. Then, a 0.035-in wire is back-loaded into the catheter so that approximately 3 to 4 cm of wire remains outside the tip of the catheter. The friction of the suture and wire against the inner sidewall of the catheter allow the suture to remain tightly fixed to the tip of the catheter. The unit as a whole can then be introduced through a 5-Fr sheath (Fig. 56.3). The tip of the wire/suture/catheter combination is advanced high within the abdominal aorta, with the opposite end of the suture lying outside of the sheath. As the catheter is pushed into the sheath and the trailing end of the suture is simultaneously pulled, the catheter folds back on itself to form (Fig. 56.4). The wire can then be withdrawn, releasing the suture, which is simply withdrawn from the sheath. The catheter is withdrawn from the sheath to engage the ipsilateral common iliac, internal iliac, and ultimately the uterine artery if desired (Fig. 56.5). A coaxial microcatheter can be advanced into the horizontal segment of the uterine artery if indicated. Following ipsilateral embolization, the catheter is advanced into the aorta and pulled down into the contralateral iliac artery for treatment of the other side.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








