chapter 5 Head and Neck
Introduction
In the United States, there are approximately 75,000 new cases of head and neck cancer each year and about 30,000 deaths due to disease.1 More than 80% of cancers in this region are squamous cell carcinoma, with the exception of tumors located in the salivary glands, thyroid, bone, and soft tissue. Because the head and neck region contains a variety of tissue types and sites, patterns of spread can vary and treatments may depend on the tumor location and stage. Diagnosis and staging are often performed with a careful physical examination, as well as endoscopic and radiologic studies. Surgery and radiation therapy, either alone or in combination, have both been used as standard treatments for squamous cell cancer in the head and neck region. More recently, chemotherapy has been used in locally advanced tumors as adjuvant therapy or in combination with radiotherapy.2–4
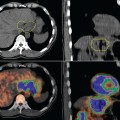
Positron emission tomography (PET) with the radiolabeled glucose analog, 18F-fluoro-deoxy-D-glucose (FDG), is currently being used increasingly in the clinic for the diagnosis and management for head and neck cancer. FDG is more readily taken up by malignant cells that incorporate the glucose analog, creating more radiointense images in a background of normal tissue. PET, however, is limited by poor anatomic detail. In contrast to computed tomography (CT) or magnetic resonance imaging (MRI), which are modalities dealing with anatomic changes, PET provides functional information that can be helpful in a region with very complex morphology. Recent advances in radiologic technology have included the integration of anatomic and functional imaging through the combined PET-CT scanner, which makes it easier to determine pathology.
Rationale for Use of PET or PET-CT in Head and Neck Cancer
Initial Staging
The use of PET in the imaging of human head and neck cancer was first reported by Schelstraete and colleagues in 1982 using nitrogen-13 (13N)-labeled ammonia.5 Haberkorn and colleagues were the first to report the use of FDG-PET for human head and neck malignancy in 1991.6 FDG-PET, CT, and MRI of the head and neck were first compared in 1992 for identification of primary and metastatic lesions of the head and neck, showing that FDG-PET was identical to or better than CT or MRI in detection of head and neck cancer.7 A more recent publication has highlighted the higher accuracy of FDG-PET in detecting the primary tumor compared to CT or MRI. Ng and colleagues correctly identified the primary tumor in 122 of 124 patients with oral cavity cancer using FDG-PET.8 CT or MRI, on the other hand, detected only 108 of 124 oral cavity primary sites, with an overall accuracy of 87.1%. In the two tumors that were missed by FDG-PET, one was a superficial tongue tumor measuring 1.0 × 1.0 × 0.1 cm; the other was a floor of mouth cancer thought to be originating from the oral tongue. There are recent data that FDG-PET does not improve detection of bone invasion in oral cavity cancer over a standard conventional CT with contrast.9
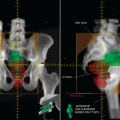
FDG-PET is more sensitive than CT or MRI in detecting occult neck metastasis. In a study of 134 oral cavity cancers, 35 (26.1%) were found to have cervical metastasis at neck dissection.10 For levels I to III lymph nodes, FDG-PET had a twofold higher sensitivity than CT or MRI. In the same study FDG-PET had a marginally lower specificity than CT or MRI in detection of cervical metastasis. Furthermore, visual correlation of both FDG-PET with CT or MRI yielded an increase in accuracy in detecting sub-clinical neck metastasis when compared to FDG-PET alone. In a study from Washington University, 20 patients with squamous cell cancer in the oral cavity, oropharynx, hypopharynx, or larynx had a PET-CT prior to neck dissection.11 PET-CT detected 17 of 17 heminecks and 26 of 27 nodal zones histologically positive by dissection. The nodal level staging sensitivity and specificity rates for PET-CT were 26 of 27 (96%) and 68 of 69 (98.5%), respectively. In contrast, CT alone had a sensitivity and specificity of 78% and 98.5%, respectively. The authors concluded that PET-CT is superior to CT alone for geographic localization of pathologic nodes.
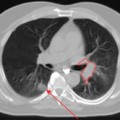
One of the advantages of obtaining a PET or PET-CT for staging is the detection of distant metastasis. Aggressive locoregional therapy can be avoided in these patients if they are found to harbor disease in distant sites. In one study, 2 of 12 patients with stage III or IV head and neck cancer by conventional staging modalities such as CT or MRI were found to have mediastinal disease on PET.12 In another study of 140 patients with nasopharyngeal cancer, FDG-PET was able to detect 26 true-positive metastatic sites in 18 patients, avoiding aggressive locoregional radiotherapy in most of these patients.13 The sensitivity and specificity of FDG-PET for distant metastasis were 100% and 86.9%. respectively. The most common sites of distant metastasis were the mediastinum (n = 8), lung, liver, and bone (n = 5 each), and distant lymph nodes (n = 3). Advanced nodal disease with N2 or N3 status was associated with an increased incidence of metastatic disease. At Emory Clinic, investigators have found 3 of 36 head and neck cancer patients to harbor distant metastasis on PET-CT that were not appreciated on CT of the head and neck or chest radiograph.14 The intent of treatment was changed from curative to palliative, avoiding a more definitive chemoradiation treatment strategy. In addition, one patient with hypopharyngeal cancer was found to have a synchronous lung primary which changed the treatment strategy. Likewise at Johns Hopkins University, 3 of 36 patients with squamous cell carcinoma of the head and neck were found on PET-CT to have distant metastasis, which otherwise would have been missed by CT or MRI of the head and neck region.15 PET-CT confirmed the CT- or MRI-based stage in 25 (69%) of patients, but altered the treatment plan in 11 mainly because of the findings of more extensive neck disease or presence of distant spread.
Surveillance
FDG-PET is excellent in identifying persistent and recurrent disease. Diminished FDG uptake appears to coincide with a decline in the number of viable tumor cells.16 Post-treatment effects, particularly with surgery and radiotherapy, make it difficult to distinguish normal post-therapy tissue changes from tumor persistence. In addition, neck dissection and flap reconstruction can distort normal anatomy and make the detection of persistent or recurrent disease difficult based on morphologic changes alone.17 Radiation therapy may cause edema and indistinct tissue planes, resulting in an increase in tissue volume and difficulty with interpretation. For these reasons, the use of functional imaging as an adjunct to either CT or MRI may be beneficial in distinguishing tumor from benign processes.
In a study from University of Pittsburg, 28 patients with head and neck cancer underwent post-treatment PET-CT after definitive radiation therapy.18 The same patients had a contrast-enhanced CT scan. The overall sensitivity and specificity for detection of residual disease were 76.9% and 93.3% for PET-CT and 92.3% and 46.7% for CT alone, respectively. The accuracy of PET-CT was 85.7% compared to 67.9% for CT alone. The investigators also found that a PET-CT performed later than 8 weeks after radiation therapy had a better accuracy rate than those performed at 4 to 8 weeks. At Wake Forest University, 12 patients with stage III or IV head and neck cancer received CT or MRI and PET before and 1 month after definitive radiotherapy.19 All patients underwent a planned neck dissection. In this small series, the presence of a positive PET 1 month after radiotherapy accurately indicated the presence of residual disease in all cases; however, a negative PET indicated absence of disease in only 14%.
At University of Iowa Hospitals and Clinics, Yao and colleagues found that a negative post-radiotherapy FDG-PET was predictive of a negative pathology in neck dissection or fine-needle aspiration.20 A total of 41 patients underwent a PET scan 2.5 to 6 months after radiotherapy, with the majority of patients receiving PET at 3 to 4 months. All patients with a negative FDG-PET after radiotherapy or those with a maximum standardized uptake value (SUV) of < 3 were found to be free of residual tumor. Using a maximum SUV value of 3 as the criterion for a negative FDG-PET scan, the negative predictive and positive predictive values were 100% and 80%, respectively. The authors postulated that a neck dissection may not be required for regional control if the postradiotherapy PET scan was negative.
Investigators from Guangzhou, China found that FDG-PET influenced salvage treatment for locally persistent nasopharyngeal carcinoma.21 In 4 of 33 patients who had histologic persistence at 1 to 6 weeks after radiotherapy, FDG-PET was negative and hence salvage treatment was not offered. All 4 patients had repeat biopsies, which were negative at a later time, and none developed a locoregional recurrence.
Unknown Primary
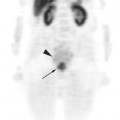
Squamous cell carcinoma of unknown primary can occur in 2% to 3% of patients presenting with cervical nodal metastasis.22 The usual workup includes a physical examination, CT scan or MRI of the head and neck; direct laryngoscopy and blind biopsies of the base of tongue, pyriform sinus; and nasopharynx and tonsillectomy. Some have found that the use of PET does not add significantly to the detection of an occult primary tumor in patients comprehensively evaluated by clinical and radiographic examinations.23,24 A more recent comprehensive review of 16 studies involving 302 patients found that FDG-PET detected primary tumors in approximately 25% of cases that were undetected by usual staging modalities.25 Furthermore, FDG-PET detected previously unrecognized regional metastasis in 15.9% and distant disease in 11.2%. In the same study, FDG-PET was found to have a low specificity for tonsillar and low sensitivity for base of tongue tumors.
Fusion Technique and Target Delineation
CT Radiotherapy Simulation
While most studies dealing with fusion of functional to CT images for radiotherapy treatment target delineation have been in non-small cell lung cancer, there have been a few reports in head and neck cancer.26 In our department at the Emory Clinic, a CT scan (with 100 mL intravenous contrast injected at a rate of 2 mL/s) in the treatment position is first performed using a light speed scanner (General Electric & Medical System, Milwaukee, WI). The planning volume is scanned at 2.5-mm increments from the top of the skull to the midthorax. Patients were scanned in the supine position using a head mask for immobilization. These CT images are later fused to the hybrid PET-CT images acquired in the nuclear medicine department.
Image Co-registration
The PET-CT datasets are sent via a Digital Imaging and Communication in Medicine (DICOM) protocol to the CT simulation workstation for image co-registration. Co-registration is performed with commercially available fusion software on the GE Advantage Sim (GE Medical Systems). Three or more reference anatomic landmarks are matched and fused with a maximal acceptable error of 5 mm. The radiotherapy CT simulation images are fused to the CT portion of the PET-CT hybrid images. These data are sent to the ECLIPSE treatment planning system (Varian, Palo Alto, CA).