chapter 13 Melanoma
INTRODUCTION
Melanoma arises from melanocytes, pigmented dendritic-like cells. Most commonly found in the skin, melanocytes can be located in various anatomic sites throughout the body. It is estimated 59,580 cases of cutaneous melanoma will be diagnosed in 2006 and that 7,770 people will die from the disease.1 Cutaneous melanoma ranks fifth in incidence among men and sixth among women of all cancer in the United States.1 However, it is the leading source of cancer death in women aged 25 to 30 years old. While most cutaneous melanomas are diagnosed at early stage due to increased awareness and screening, 6% to 10% of patients show detectable metastases at the time of diagnosis and another 16% develop metastases after surgical resection.2,3
FDG-PET in Melanoma
FDG-PET (18F-fluoro-deoxy-D-glucose-positron emission tomography) imaging in melanoma has been extensively studied, with reviews by several authors.4–6 It has been shown to have a high sensitivity and specificity for melanoma, particularly for staging workup. As reviewed by Friedman and Wahl,4 the sensitivity and specificity of FDG-PET in detecting metastatic disease in patients with melanoma ranges from 71% to 100% and 56% to 100%, respectively. Tyler and colleagues7 prospectively studied 95 patients with clinically evident stage III disease; results demonstrated a sensitivity of 87.3% for FDG-PET in detecting histologically confirmed melanoma sites. In a smaller group of stage III patients, Acland and colleagues8 also found a high sensitivity of 93% for FDG-PET in detection of melanoma sites; unexpected distant metastases were detected in 28.5% of patients.
Detection of melanoma with FDG-PET imaging is dependant upon tumor volume. Crippa and associates9 showed only 23% of the nodal disease was detected in lymph nodes measuring 5 mm or less compared to 83% for nodes between 6 to 10 mm. Wagner and colleagues10 recently reported a prospective study comparing FDG-PET with sentinel lymph node biopsies in 144 subjects with clinically localized melanoma. Results showed lymph node tumor volume of < 80 mm3 was detected in only 4 of 37 basins with a sensitivity of 11%; detection improved in volumes > 80 mm3, detecting disease in 4 of 5 basins with a sensitivity of 80%. Even with these results, FDG-PET is still superior to conventional imaging. Swetter and associates11 compared FDG-PET to body computed tomography (CT) (chest, abdomen, and pelvis) in 104 patients with melanoma, the majority of whom had stage III or IV disease. The sensitivity and specificity of PET in diagnosis metastatic disease was 84% and 97%, respectively, compared to the 58% and 70%, respectively for CT. When anatomical sites not routinely imaged with CT were excluded from the analysis, sensitivity and specificity of CT increased only marginally, reported at 69% and 70%, respectively. The superiority of FDG-PET over CT was also demonstrated by Holder and coworkers12 in 103 patients with stage II through IV melanoma, with a reported sensitivity and specificity of 94% and 83%, respectively, for FDG-PET contrasted to 55% and 84%, respectively, for CT. Of the 9 false-positive cases in these series 4 had other tumors (2 malignant, 2 benign) and 5 were due to post-surgical or inflammatory conditions. Rinne and colleagues13 compared FDG-PET to a battery of conventional imaging studies, including CT of the thorax and abdomen, abdominal ultrasound, and magnetic resonance imaging (MRI) of the brain in 100 patients considered to have high-risk melanomas. Again, sensitivity and specificity of FDG-PET was higher than conventional imaging modalities, with 92% and 94%, respectively, compared to 58% and 45%, respectively, for conventional imaging.
The improved accuracy of FDG-PET has been shown to have a significant impact on management of patients with advanced melanoma. Gulec and coworkers14 prospectively imaged 49 consecutive patients with clinically suspected or diagnosed metastatic melanoma with FDG-PET and conventional imaging included CT of the chest, abdomen, and pelvis and an MRI of the brain. A treatment plan was initially formulated based on conventional imaging and subsequently when PET data were available. FDG-PET identified more metastatic sites than conventional imaging in 55% of patients. The treatment plan was altered for 49% of patients: changing treatment modality from surgical to systemic therapy (25%), adding operative procedures (12%), and adding systemic treatment or radiotherapy (12%). The clinical impact of FDG-PET was recently confirmed in a retrospective analysis of a larger patient population (n = 254) where PET upstaged 22% of the patients.15 Treatment was changed, usually from surgery to systemic therapy, in 17% of patients who presented with stage III disease.
PET-CT in Melanoma
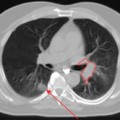
PET-CT fusion images help locate PET abnormalities when CT fails to detect an abnormality in the same area. However, there are no systematic studies comparing PET-CT and PET alone in melanoma. Schoder and colleagues16 reported a patient with melanoma who complained of back pain. FDG-PET showed irregular, intense uptake in midthoracic spine; the corresponding CT image showed increased paravertebral soft tissue. PET-CT fusion images demonstrated metastasis in right paravertebral region with extension through neural foramen into the spinal canal. In addition, the authors also reported a case that used PET-CT to localize the FDG uptake focus to the third portion of duodenum; a third case reported with a focus localized to the gallbladder. In both cases, CT revealed no abnormality in the area and PET alone was difficult to use for accurate localization. Finkelstein and colleagues17 reported a prospective study on stage IV patients with melanoma imaged with conventional imaging (CT and/or MRI) and PET before a planned metastasectomy. This study demonstrated the combination of PET and conventional imaging was more sensitive and specific than either modality alone, with a sensitivity and specificity of 88% and 91% for combined reading, compared to 79% and 87% for PET alone and 76% and 87% for conventional imaging alone. Interestingly, sensitivity increased significantly compared to specificity, presumably because the CT abnormalities alerted a more careful assessment of FDG-PET in areas of concern. Although no formal comparison data exist in the literature, these findings strongly suggest the accuracy of PET will be improved with the use of integrated PET-CT scanners.
PET-CT in Radiation Treatment Planning in Melanoma
Cutaneous Melanoma
Cutaneous melanoma is treated with complete surgical resection. Adjuvant radiation has the potential to improve local control for patients who have a high risk of local recurrence after surgery. High-risk features include head and neck desmoplastic primaries, thick or ulcerated non-desmoplastic primaries, close or positive resection margins, and locally recurrent disease.18
Cutaneous melanoma has a high risk of regional lymph node metastasis, dependant upon the depth of invasion. It is estimated 30% of lymph node metastasis occurs when the depth of invasion is > 2 mm; up to 50% when the depth of invasion is > 4 mm.19
Neck dissection as a solitary treatment for patients with lymph node metastasis has a high regional recurrence, particularly for patients with extracapsular extension, 3 or more involved nodes, or a node size of > 3 cm. For patients with these features, regional failure rates ranging from 30% to 50% are reported with neck dissection alone.20,21 Adjuvant radiation after neck dissection is recommended; locoregional control rates of 82% to 95% were achieved in several studies, although survival rates did not improve compared to neck dissection alone.22–25
Mucosal Melanoma
Primary mucosal melanoma is rare. In the National Cancer Database report on more than 84,000 cases of melanomas, only 1.3% were noted to arise from mucosal surfaces.26 While mucosal melanoma can occur in the anus, rectum, vulva, and vagina, more than half are located in the head and neck region. Of a thousand cases of primary head and neck mucosal melanoma reported in the literature through 1997, the oral cavity is the most common site for the primary lesion, followed by the nasal cavity and the paranasal sinuses.27 Within the oral cavity, the most frequent primary sites are the palate and the maxillary gingiva, accounting for 80% of all oral melanomas. Other oral sites include the mandibular gingiva, buccal mucosa, tongue, and floor of the mouth. Within the nasal cavity, the lower septum and the middle and inferior turbinates are the most affected sites. The maxillary sinus is the most common primary site in the paranasal sinus.28,29
The prognosis of mucosal melanoma is poor, with a 5-year survival rate of < 20%. As in cutaneous melanoma, the primary treatment for head and neck mucosal melanoma is complete surgical resection. The role of postoperative radiation is controversial. However, given the high incidence of local recurrence and anatomical limitation of radical surgery, postoperative radiation is generally recommended in patients with high-risk features, including large tumors, close or positive surgical margins, perineural invasion, and nasal cavity or paranasal location.30–32 Because of the rarity of mucosal melanoma, it is impossible to carry out randomized studies. However, several retrospective studies have confirmed the role of postoperative radiation in improving local control. Owens and colleagues30 reviewed 48 head and neck mucosal melanoma cases treated at The M.D. Anderson Cancer Center from January 1985 through December 1998. The review revealed that 9 of 20 patients (45%) who had surgery alone failed locoregionally, while only 4 of 24 patients (17%) who had postoperative radiation failed locally. Temam and colleagues31 reported on 69 patients treated at the Institut Gustave-Roussy: local control rates were 26% with surgery alone (8 of 30) and 62% (24 of 39) when including post-operative radiation.
For patients with unresectable or inoperable head and neck mucosal melanoma, radiation treatment as a primary treatment can also been applied. Gilligan and Slevin33 reported 28 cases of mucosal melanoma in the nasal cavity and paranasal sinuses treated with definitive radiation. Initial complete regression was observed in 22 patients (79%) and absolute local control by radiotherapy alone was achieved in 17 patients (61%). Follow-up was limited in many cases by early death due to metastatic disease; actuarial local disease-free survival was 49% at 3 years. The authors concluded radical radiotherapy for mucosal melanoma of this site can be justified on the basis of the local control achieved and low treatment morbidity in patients who are typically elderly and the propensity to disseminated disease. Wada and colleagues34 reported 31 patients with head and neck mucosal melanoma treated by radiation, 21 of them by radiation alone and 10 with gross residual tumor after surgery. Complete response was achieved in 9 patients (29%) and partial response (defined as tumor volume decrease by > 50% after treatment) in 18 patients (58%). Thirteen patients (41.9%) had local recurrences. The authors reported the dose per fraction and biologically equivalent dose were significantly related to both local control and cause-specific survival.
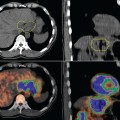
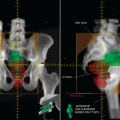
In post-operative radiation treatment, PET-CT plays only a limited role for target delineation and treatment planning, as is the case in cutaneous melanoma. However, it could be very useful for definitive radiation for mucosal melanoma. This is illustrated in an 83-year female patient with sphenoid sinus melanoma. She presented with right eye diplopia, right ptosis, and right cranial nerve III palsy. CT and MR scans revealed a mass in the right side of the sphenoid sinus with extension into the cavernous sinus. The FDG-PET revealed intense uptake of FDG by the mass with a maximal standardized uptake value (SUV) of 18. An endoscopic biopsy of the mass revealed melanoma. The tumor was deemed unresectable because of the location and the nerve involvement. The patient received definitive radiation with intensity-modulated radiotherapy (IMRT)35 with optical stereotactic guidance.36
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree