Persistent disease (n = 24)
Recurrent disease (n = 24)
All (n = 48)
Gender
Male (n)
17
18
35
Female (n)
7
6
13
Age
<50 (n)
14
14
28
≥50 (n)
10
10
20
Median (years)
45
47
46
Range (years)
32–86
35–84
32–86
rT classification
rT1 (n)
18
9
27
rT2 (n)
2
4
6
rT3 (n)
4
7
11
rT4 (n)
0
4
4
Prior local failures
Yes (n)
0
9
9
No (n)
24
15
39
Previous salvage Rx
Surgery (n)
0
4
4
ERT (n)
0
7
7
Radiosurgery (n)
0
1
1
Cumulative ERT dose
65–68 Gy (n)
22
16
38
70–76 Gy (n)
2
1
3
125–130 Gy (n)
0
7
7
Interval from end of first ERT
≤6 months (n)
24
0
24
>6 months to 1 year (n)
0
4
4
>1–2 years (n)
0
3
3
>2–3 years (n)
0
5
5
>3 years (n)
0
12
12
Median (months)
4
36
8
Range (months)
3–6
9–197
3–197
Prior to radiosurgery, all patients were restaged by physical examination, nasopharyngoscopy and biopsy, and CT of nasopharynx and neck. Patients with disease confined to nasopharynx or with limited extension to nasal fossa, parapharyngeal space, and oropharynx were first evaluated jointly by a surgeon and a radiation oncologist for brachytherapy or surgery, and radiosurgery was offered only to those patients whose disease was deemed not amenable to these salvage treatments. At our institution, brachytherapy for persistent or recurrent NPC was usually performed using gold grain implantation. This technique was applicable only to patients with a well-defined and relatively small-volume tumor confined to the nasopharynx. Patients with extensive mucosal recurrence involving a large area were not suitable for gold grain implantation. In addition, patients with relapse at the cushion near the opening of eustachian tube were also not suitable for the procedure because gold grain could not be directly implanted into the relatively thin mucosa overlying the cartilaginous cushion. In general, patients with small-volume mucosal tumor were usually treated by gold grain implantation, with surgery reserved for those with more bulky tumor or those with relapse at the cushion. Patients with disease amenable to surgery or brachytherapy who refused these treatments or were considered to be medically contraindicated for these procedures were also offered radiosurgery.
Target Localization
Tumor extent was assessed by imaging and endoscopic examination before radiosurgery. For imaging study, axial contrast CT with a slice thickness of 2.5–3 mm was performed in all patients, supplemented by axial contrast MRI with a slice thickness of 3 mm in 73 % of patients. PET-CT was not performed in this patient group for target localization, although we are currently also evaluating its role in salvaging local failures of NPC. Target volume was defined as any abnormal soft tissue mass and/or contrast-enhancing areas as shown in axial imaging plus a margin of about 2–3 mm. Endoscopic examination was always performed irrespective of imaging findings to assess the mucosal extent of tumor, including any spread to nasal cavity or oropharynx. In our experience, endoscopic examination is the most reliable means to access the mucosal extent of disease and should not be omitted even when imaging showed that only part of the nasopharynx was involved. During endoscopy, multiple biopsies were taken from both sides of the nasopharynx to assess the macroscopic as well as microscopic extent of tumor. In patients just completing primary radiotherapy, biopsies were routinely taken from bilateral roofs, lateral walls, and posterior walls. In patients with tumor apparently localized to one part of the nasopharyngeal mucosa, mapping of tumor extent was performed by taking multiple biopsies from grossly uninvolved mucosa surrounding the tumor. The results of mapping were then used to guide the extent of target coverage for radiosurgery.
Radiosurgery Planning and Treatment
Radiosurgery was performed using the commercial XKnife system (Radionics, Burlington, MA) to deliver multiple non-coplanar arcs of photon to the target with a modified 6-MV linear accelerator (Clinac 600C; Varian, Milpitas, CA). Head immobilization and target localization were performed with the Brown–Roberts–Wells head frame and stereotaxic system (Radionics). The head frame was anchored by placing two anterior head pins to the forehead and two to the occiput.
The head frame was not placed in a strict horizontal position but with the posterior side tilted downward to ensure that the localizer ring would include the whole nasopharynx. Using this approach, it was possible to treat the entire nasopharynx and adjacent soft tissues, although special attention should be given to those with disease extending down to the junction of the oropharynx. Although some investigators adopted the approach of anchoring the head ring anteriorly to the zygomas to ensure complete coverage of the nasopharynx, we did not find this to be necessary for most patients. The target volume was localized as described above. For rT1 tumor, target confined to one side of nasopharynx was usually covered using one isocenter, whereas target involving both sides was covered using one or two isocenters. For rT2–4 tumors, target was usually covered by one or two isocenters. We found it seldom necessary to use more than two isocenters. The target volume was treated using 3–5 arcs of beams with a degree of 90–210°. The following dose limits were set to the critical structures: 5 Gy to brain stem, 4 Gy to optic apparatus, and 5 Gy to temporal lobes. An additional dose limit of 8 Gy to internal carotid artery was later set to reduce the risk of hemorrhage. Most patients received a dose of 12.5 Gy delivered to the 80 % isodose line. Table 41.2 summarizes the radiosurgery treatment.
Table 41.2
Summary of radiosurgery treatment parameters of patients treated at Queen Mary Hospital, Hong Kong
Parameters | Persistent disease | Recurrent disease | All |
|---|---|---|---|
Number of isocenters | |||
One (n) | 20 | 19 | 39 |
Two (n) | 4 | 4 | 8 |
Three (n) | 0 | 1 | 1 |
Size of collimator | |||
≤20 mm (n) | 4 | 1 | 5 |
>20–30 mm (n) | 19 | 16 | 35 |
>30 mm (n) | 1 | 7 | 8 |
Median (mm) | 25 | 30 | 25 |
Range (mm) | 12.5–35 | 15–37.5 | 12.5–37.5 |
Target volume | |||
<10 cm3 (n) | 23 | 15 | 38 |
≥10 cm3 (n) | 1 | 9 | 10 |
Median (cm3) | 4.0 | 5.7 | 4.6 |
Range (cm3) | 1.3–14.5 | 2.7–30.7 | 1.3–30.7 |
Prescribed dose to 80 % isodose line | |||
<12 Gy (n) | 3 | 5 | 8 |
12–13 Gy (n) | 17 | 13 | 30 |
>13 Gy (n) | 4 | 6 | 10 |
Median (Gy) | 12.5 | 12.5 | 12.5 |
Range (Gy) | 11.1–18 | 8–14 | 8–18 |
Dose to isocenter | |||
<15 Gy (n) | 4 | 5 | 9 |
15–16 Gy (n) | 16 | 13 | 29 |
>16 Gy (n) | 4 | 6 | 10 |
Median (Gy) | 15.6 | 15.6 | 15.6 |
Range (Gy) | 13.9–22.5 | 10–17.5 | 10–22.5 |
Tumor Control After Radiosurgery
Thirty-seven (77 %) patients achieved complete regression of tumor after radiosurgery. All patients (100 %) treated for persistent disease achieved complete regression of disease compared with 13 (54 %) patients for recurrent disease. Local failure after radiosurgery occurred in 22 (46 %) patients, regional failure in 5 (10 %) patients, and distant metastasis in 7 (15 %) patients. For those patients who failed locally after radiosurgery, two received brachytherapy with intubation and both were salvaged, four had nasopharyngectomy and three were salvaged, and five had external radiotherapy and two were salvaged. One patient with local failure outside the radiosurgery-treated volume was salvaged by second radiosurgery. Three-year local relapse-free and overall survival rates for all patients were 52 % and 66 %, respectively. Patients treated for persistent disease had better outcome than those treated for recurrent disease: 3-year local relapse-free survival rate was 76 % in the former and 29 % in the latter (Fig. 41.3), and the corresponding 3-year overall survival rates were 81 and 54 % (Fig. 41.4). Patients with advanced T classification at the time of relapse had a poorer control rate after radiosurgery: 3-year local relapse-free survival rates were 69 % for rT1 disease, 40 % for rT2–3 disease, and 0 % for rT4 disease (Fig. 41.5). Patients with bulky tumor also had a poorer control rate after radiosurgery: 3-year local relapse-free survival rate was 61 % for target volume <10 cm3 compared with 20 % for target volume ≥10 cm3 (Fig. 41.6).







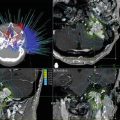
Fig. 41.1
(a) Baseline CT of a patient with recurrent T1 nasopharyngeal carcinoma showing mass at left lateral wall of nasopharynx (arrow). (b) Planning CT showing isodose distribution of target covered by single isocenter. The prescribed dose to target periphery was 12.5 Gy. (c) Follow-up CT taken 12 months after radiosurgery showing complete resolution of tumor

Fig. 41.2
(a) Baseline CT of a patient with recurrent T2 nasopharyngeal carcinoma showing mass lesion at right side of nasopharynx extending to nasal fossa. (b) Planning CT showing isodose distribution of target covered by two isocenters. The prescribed dose to target periphery was 14 Gy. (c) Follow-up CT taken 6 months after radiosurgery showing complete resolution of tumor

Fig. 41.3
Comparison of local relapse-free survival curves in patients with persistent and recurrent nasopharyngeal carcinoma treated by stereotactic radiosurgery at Queen Mary Hospital

Fig. 41.4
Comparison of overall survival curves in patients with persistent and recurrent nasopharyngeal carcinoma treated by stereotactic radiosurgery at Queen Mary Hospital

Fig. 41.5
Comparison of local relapse-free survival curves according to recurrent T classification after stereotactic radiosurgery for local failures of nasopharyngeal carcinoma at Queen Mary Hospital

Fig. 41.6
Comparison of local relapse-free survival curves according to tumor volume after stereotactic radiosurgery for local failures of nasopharyngeal carcinoma at Queen Mary Hospital
Complications
Radiosurgery was well tolerated, and we did not observe any acute complications. In assessing late effects after treatment, it was not possible to attribute the cause of complication to radiosurgery or external radiotherapy. Some patients also received another course of external radiotherapy either before or after radiosurgery, and they would expect to have a relatively high incidence of late complications even without radiosurgery. Table 41.3 summarizes the late complications observed in our patients, which included all observed complications (other than xerostomia) irrespective of whether they were thought to be mainly caused by radiosurgery or not. Overall, 31 % of patients developed one or more late complications. The percentage decreased to 24 % if patients with two courses of radiotherapy before radiosurgery were excluded, and it further decreased to 19 % if those with a second course of radiotherapy before or after radiosurgery were excluded. Patients with more advanced tumor tend to have a higher risk of developing late complications. The percentages of patients who developed late complications were 19 % in rT1 disease, 33 % in rT2 disease, 46 % in rT3 disease, and 75 % in rT4 disease. Common late complications observed were cranial neuropathy and temporal lobe necrosis, both occurring in about 15 % of patients. Two patients developed severe epistaxis, and both were found to have internal carotid artery aneurysm located at cavernous sinus and retropharyngeal space. Stenting was performed in both patients, which successfully controlled the bleeding, and there were no treatment-related deaths.
Table 41.3
Late complications after radiosurgery for locally recurrent nasopharyngeal carcinoma at Queen Mary Hospital, Hong Kong
Complication | One course of ERT (n = 36) | Two courses of ERT (n = 12) | One to two courses of ERT (n = 48) |
|---|---|---|---|
Cranial neuropathy | 2 | 5 | 7 |
Temporal lobe necrosis | 4 | 3 | 7 |
Hypopituitarism | 1 | 1 | 2 |
Carotid artery aneurysm | 1 | 1 | 2 |
Trismus | 0 | 1 | 1 |
None | 29 | 4 | 33 |
Radiosurgery Treatment Results for Local Failures of NPC in Other Series
Radiosurgery Alone
Firlik et al. first reported the use of Gamma Knife radiosurgery in a patient with recurrent NPC and noted complete regression of tumor after delivering a dose of 20 Gy to 50 % isodose line [22]. Miller et al. reported another three patients with NPC that were also treated by Gamma Knife: one patient was noted to have symptomatic improvement before disease progression, and one patient had not responded to the treatment [23]. Buatti et al. treated three patients with recurrent NPC by Linac radiosurgery using a dose of 12.5 Gy to 80 % isodose line [24]. Two patients also received reirradiation, one before and the other after radiosurgery. Of these two patients, one remained disease-free 1 year after treatment, whereas the other had local recurrence 6 months after treatment. The third patient received radiosurgery alone for recurrent disease and had neurologic deterioration of uncertain etiology 6 months after the treatment. Kocher et al. treated five patients with recurrent NPC at the skull base using radiosurgery with a dose of 15–24 Gy [25]. Only one patient’s tumor was controlled after treatment. Three patients developed late complications, including two with fatal internal carotid artery hemorrhage. Cmelak et al. reported using Linac radiosurgery in treating 12 recurrent NPC lesions in nine patients [26]. The dose delivered ranged from 15 to 20 Gy with a median of 18 Gy. With a median follow-up of 17 months, the crude local control was 53 % (7/12), and only one patient developed radiation-induced cranial neuropathy.
Radiosurgery as a Boost After External Reirradiation
Chen et al. reported the outcome of 11 patients with rT3–4 NPC after conformal radiotherapy and Linac radiosurgery [27]. The radiosurgery dose ranged from 10 to 19 Gy with a median of 14 Gy. Significant regression of tumor was noted in five patients and limited regression in another three. Chang et al. reported 15 patients with locally recurrent NPC who received external reirradiation followed by radiosurgery using a dose ranging from 8 to 15 Gy [18] and noted a 3-year survival rate of 52 %. Pai et al. reported 36 patients with recurrent NPC also treated with external reirradiation followed by radiosurgery boost [28]. The radiosurgery dose to target periphery ranged from 8 to 20 Gy with a median of 12 Gy. A 3-year local control rate of 58 % was achieved. In these reports, it is uncertain whether radiosurgery was used to boost the entire or partial tumor volume, with the intention to further escalate the total dose or to selectively boost the volume that was underdosed during external reirradiation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree