Magnetic resonance (MR) imaging is superior to ultrasonography (US) for the evaluation and prognostication of neonates with neonatal encephalopathy (NE). Nonetheless, US may provide important information early in the course of NE and can be used to document the evolution of lesions. This article provides an overview of useful findings in the US evaluation of infants with NE. Although many of the findings do not appear as conspicuous or as extensively as they do on MR imaging, recognition and familiarity with subtle head US abnormalities may allow head US to play an important complementary role to MR imaging in the evaluation of infants with NE.
Neonatal encephalopathy (NE) is a major cause of mortality and morbidity in newborns. NE occurs in 1 to 6 per 1000 live full-term births and is the most important cause of brain damage in the newborn. The consequences are potentially devastating, because it can lead to mortality or severe disability.
This article discusses and illustrates the ultrasound (US) findings present in infants with NE and correlates them with the findings present on MR imaging when appropriate. Although MR imaging has become more widely used and has gained widespread acceptance in the evaluation of NE in recent years, US continues to be the primary screening imaging modality used for the evaluation of the neonatal brain. In many neonates, the diagnosis of brain abnormalities has been made using cranial US. It is a noninvasive, inexpensive, and portable imaging modality that allows examinations to be performed without requiring transport of the infant. The anterior fontanelle provides a convenient sonographic window that allows excellent imaging of many regions of the brain.
Throughout the article, the clinical use of linear US images that are obtained with high-frequency linear array transducers is emphasized. Also discussed is how Doppler imaging and additional windows can be used to improve diagnostic accuracy. Linear US images are a useful adjunct to conventional US and enable further characterization of the architecture of the brain parenchyma, pathologic conditions, and in some instances more precise anatomic localization and diagnoses.
Clinical features of NE
NE is clinically defined as a “syndrome of disturbed neurologic function in the earliest days after birth in the term infant, manifested by difficulty with initiating and maintaining respiration, depression of tone and reflexes, subnormal level of consciousness and often seizures” ; whereas hypoxic–ischemic injury (HII) is defined as NE with intrapartum hypoxia in the absence of any other abnormality. HII is characterized by a distinctive encephalopathy that evolves from lethargy to hyperexcitability to stupor during the first 3 days of life. Neonates with HII are usually born at term, but the condition also occurs in premature neonates. HII may be difficult to diagnose in premature infants, especially those with very low birth weight, because the obvious signs are absent or because the symptoms that are present are attributed to developmental immaturity. Involvement of multiple additional organs is a characteristic feature of HII. HII may present with multiorgan dysfunction and severely depressed cardiovascular function, particularly if it is severe. Patients may have severe pulmonary hypertension requiring assisted ventilation. Renal (eg, anuria or oliguria), hepatic (eg, abnormal liver function tests), and gastrointestinal injury (eg, delayed gastric emptying and poor peristalsis) are also common, although they do not occur immediately. Notably, the presence of renal dysfunction in association with an abnormal neurologic clinical examination in infants with HII is associated with a poor long-term neurodevelopmental outcome.
According to the guidelines of the American College of Obstetricians and Gynecologists (ACOG) and the American Academy of Pediatrics (AAP), the following four criteria must be present for a diagnosis of HII to be made: (1) evidence of metabolic acidosis (pH <7.0 and a base deficit of ≥12 mmol/L); (2) early onset of severe or moderate NE in infants born at greater than or equal to 34 weeks’ gestation; (3) quadriplegic or dyskinetic cerebral palsy (CP); and (4) exclusion of other identifiable etiologies, such as trauma, infection, a coagulation disorder, or a genetic disorder. However, although CP is frequently attributed to asphyxia, most cases of CP are not associated with severe perinatal asphyxia, and vice versa not all cases of perinatal asphyxia result in CP. It is also believed that some infants who develop HII may have experienced asphyxia or brain hypoxia remote from the time of delivery but exhibit the signs and symptoms of hypoxic encephalopathy at the time of birth.
More than 10 years ago, a case-control study performed in Western Australia concluded that there was no evidence of intrapartum asphyxia in more than 70% of cases of NE. They found that the causes of NE are heterogeneous and many occur before birth. The antepartum risk factors for NE include (1) low socioeconomic status; (2) advanced maternal age; (3) a family history of seizures; (4) maternal thyroid disease; (5) maternal hypertension; (6) vaginal bleeding; (7) conception after infertility treatment; (8) in utero viral illness; (9) abnormal placenta; (10) postterm delivery; and (11) intrauterine growth restriction, with the latter being the strongest risk factor. Only a small fraction of NE is caused by HII, and it has been associated with intrapartum risk factors, such as forceps delivery, breech extraction, cord prolapse, abruptio placentae, and maternal fever.
In 2003, Cowan and colleagues found that more than 90% of 360 term infants without known genetic syndromes or major congenital defects who underwent MR imaging within the first 2 weeks of life and who developed NE, seizures, or both had MR imaging evidence of brain injury at or near the time of delivery. However, these authors could not distinguish between brain injuries acquired during versus just before delivery.
More recently, several investigators have emphasized the importance of maternal infection during pregnancy as a major risk factor for CP in term and preterm infants. In a review by Graham and colleagues published in 2008, the authors concluded that despite the existence of objective criteria for grading of NE, investigators often use different definitions of intrapartum HII, which results in variations in different institutions’ rates of NE. Nonetheless, regardless of the definition applied, the incidence of HII in developed countries was 2.5 per 1000 live births, and only 14.5% of CP cases in the reviewed studies were associated with intrapartum hypoxia–ischemia. Therefore, the term NE is favored over HII unless all of the ACOG and AAP criteria are known to be met. It also should be noted that the cause of CP remains incompletely understood.
Patients’ clinical manifestations and course vary depending on the severity of their NE. Although several scoring systems exist, the staging system created by Sarnat and Sarnat is the most widely used. It recognizes three stages of NE and correlates them with clinical outcomes. At one end of the spectrum, Sarnat stage 1, or mild encephalopathy, is characterized by irritability, poor feeding, hyperreflexia, and an exaggerated Moro reflex and has no long term-sequelae. On the other end of the spectrum, patients with Sarnat stage 3, or severe encephalopathy, have seizures and usually require ventilatory support. These patients usually have poor outcomes.
Neuropathologic features of perinatal brain injuries
There are four major patterns of hypoxic–ischemic brain lesions: (1) parasagittal brain injury, (2) periventricular leukomalacia (PVL), (3) selective neuronal necrosis (SNN), and (4) focal or multifocal ischemic brain lesions.
Parasagittal Brain Injury
This condition usually occurs in term neonates. It is classically bilateral, symmetric, and affects the parasagittal portions of the cerebral convexities corresponding to the “watershed” areas between the territories of the anterior, middle, and posterior cerebral arteries. This type of damage is associated with chronic, possibly repetitive ischemic insults ; mild to moderate hypotension ; and reflects the so-called “prolonged partial asphyxia.” In these cases, brain injury occurs secondary to insufficient perfusion to the watershed areas between the main cerebral arteries during periods of ischemia. This type of injury mainly affects the motor cortex (especially the portion responsible for proximal extremity function) and its clinical manifestations tend to include seizures, hypotension, or both. The upper extremities are often more severely affected than the lower extremities. These patients present with spastic quadriplegia and seizure disorders later in life. Their cognitive outcome is unpredictable.
Periventricular Leukomalacia
PVL is the most common injury in preterm neonates and is caused by the anatomic characteristics of the brain’s circulation related to gestational age. Before 32 weeks of gestation, blood vessels penetrate the cortex from the pial surface. Fetuses of this age have short penetrators (which end in the subcortical white matter) and long penetrators (which extend deeper into the brain). This results in relatively poor vascularization of the periventricular white matter, which predisposes premature infants to ischemic injury. The areas that are the most prone to damage include the periventricular white matter that is dorsal and lateral to the external angles of the lateral ventricles, particularly the centrum semiovale and the optic (trigone and occipital horns) and acoustic (temporal horn) radiations. Because the lower-extremity axons of the corticospinal tract, which are periventricular in location, course medially to upper-extremity axons, these patients present later in life with spastic diplegia (ie, impaired motor function that affects the lower extremities most severely). Visual field disorders are also characteristic of PVL because of damage that occurs within the optic radiations. With more severe brain injury, spastic quadriplegia with associated visual and cognitive deficits may also be observed.
Selective Neuronal Necrosis
SNN is the most common pattern associated with hypoxic–ischemic events and usually coexists with all of the other patterns. The site of the injury depends on the severity of the insult and gestational age of the neonate. The long-term sequelae of this type of injury include mental retardation, spastic quadriparesis, and seizures. Choreoathetosis and dystonia may be seen if the thalamus and basal ganglia are involved. Of note, the basal ganglia seem to be particularly sensitive to hypoxia. Bulbar and pseudobulbar palsy can occur if the brainstem and tegmentum are affected. Hypoperfusion with subsequent reperfusion injury and glutamate-induced injury are involved in the pathogenesis of SNN. The four major patterns of injury include (1) diffuse, (2) cerebral cortex–deep gray matter, (3) deep gray matter–brainstem, and (4) pontosubicular injury.
Diffuse neuronal injury
This type of SNN occurs after severe, very prolonged hypoxic–ischemic insults in both term and premature infants and affects nearly all neurons in the neuroaxis. It most frequently affects the cortex, hippocampus, cerebellum, and anterior horn cells of the spinal cord. Within the cortex, the injury is more prominent in the watershed zones and more marked in the depth of the sulci than in the gyri. With more severe injuries, the more differentiated visual (calcarine) cortex and the perirolandic cortex may be damaged.
Cerebral cortex–deep gray matter
This type of SNN occurs mainly in term neonates after moderate to severe, usually prolonged hypoxic–ischemic insults. Injury is generally bilateral and typically affects the dorsolateral putamen and ventrolateral thalami. This combination is typical of HII in the term neonate.
Deep gray matter–brainstem
This type of SNN occurs mainly in term neonates after severe, prolonged insults that are fairly abrupt in onset. Although injury to the basal ganglia and thalamus occurs in roughly 75% of these patients, only 15% to 20% of neonates with HII exhibit involvement of the deep gray matter and brainstem as the prevailing lesion that is present, and only a portion of these evolve to “status marmoratus.” Status marmoratus is so-named because of the marbled appearance acquired by the affected structures (particularly the putamen) after this type of injury. It is characterized by neuronal loss, gliosis, and hypermyelination. The complete pathologic picture is not apparent until at least 8 months after birth, although the insult occurs in the perinatal period. The clinical condition manifests later in life with cognitive deficits and movement disorders, including choreoathetosis and dystonia, which may not be apparent until 1 to 4 years of age.
Pontosubicular injury
This type of SNN affects the neurons of the basis pontis and the subiculum of the hippocampus. This type of injury occurs mainly in preterm neonates and is strongly associated with PVL. Clinically, it is associated with hypoxia–ischemia, hypocarbia, and hyperoxia.
Focal or Multifocal Ischemic Brain Lesions
This form of injury is unusual before the 28th week of gestation. Its incidence increases with gestational age and most commonly occurs as a result of postnatal events. It mainly affects the middle cerebral artery territory, and it affects the left middle cerebral artery more often than it affects the right. When venous structures are affected, superior sagittal sinus thrombosis is the most common causative lesion. The major causes of these lesions include perinatal asphyxia, infection, trauma, and coagulation disorders. However, the etiology of this type of injury remains unknown in roughly half of patients.
Differentiation between these various types of brain lesions, especially between focal or multifocal necrosis, parasagittal injury, and PVL, can be challenging, and in many cases these patterns of injury coexist.
At the molecular level, it has recently been postulated that HII occurs in two steps in the mature fetal brain: ischemia and reperfusion. The first is believed to be the result of an acute reduction in umbilical or uterine circulation. Recently, it has been postulated that a considerable proportion of neuronal injury occurs during the second phase of neuronal cell damage, the reperfusion phase. This is believed to result from several different types of inflammatory reactions in conjunction with the inhibition of protein synthesis, which eventually leads to the induction of neuronal apoptosis (programmed cell death). There are also increasing data showing an association between HII and ascending intrauterine infections either before or after birth, possibly caused by endotoxin-mediated cytokine release. Furthermore, it is believed to be a synergistic effect between infection and HII.
Neuropathologic features of perinatal brain injuries
There are four major patterns of hypoxic–ischemic brain lesions: (1) parasagittal brain injury, (2) periventricular leukomalacia (PVL), (3) selective neuronal necrosis (SNN), and (4) focal or multifocal ischemic brain lesions.
Parasagittal Brain Injury
This condition usually occurs in term neonates. It is classically bilateral, symmetric, and affects the parasagittal portions of the cerebral convexities corresponding to the “watershed” areas between the territories of the anterior, middle, and posterior cerebral arteries. This type of damage is associated with chronic, possibly repetitive ischemic insults ; mild to moderate hypotension ; and reflects the so-called “prolonged partial asphyxia.” In these cases, brain injury occurs secondary to insufficient perfusion to the watershed areas between the main cerebral arteries during periods of ischemia. This type of injury mainly affects the motor cortex (especially the portion responsible for proximal extremity function) and its clinical manifestations tend to include seizures, hypotension, or both. The upper extremities are often more severely affected than the lower extremities. These patients present with spastic quadriplegia and seizure disorders later in life. Their cognitive outcome is unpredictable.
Periventricular Leukomalacia
PVL is the most common injury in preterm neonates and is caused by the anatomic characteristics of the brain’s circulation related to gestational age. Before 32 weeks of gestation, blood vessels penetrate the cortex from the pial surface. Fetuses of this age have short penetrators (which end in the subcortical white matter) and long penetrators (which extend deeper into the brain). This results in relatively poor vascularization of the periventricular white matter, which predisposes premature infants to ischemic injury. The areas that are the most prone to damage include the periventricular white matter that is dorsal and lateral to the external angles of the lateral ventricles, particularly the centrum semiovale and the optic (trigone and occipital horns) and acoustic (temporal horn) radiations. Because the lower-extremity axons of the corticospinal tract, which are periventricular in location, course medially to upper-extremity axons, these patients present later in life with spastic diplegia (ie, impaired motor function that affects the lower extremities most severely). Visual field disorders are also characteristic of PVL because of damage that occurs within the optic radiations. With more severe brain injury, spastic quadriplegia with associated visual and cognitive deficits may also be observed.
Selective Neuronal Necrosis
SNN is the most common pattern associated with hypoxic–ischemic events and usually coexists with all of the other patterns. The site of the injury depends on the severity of the insult and gestational age of the neonate. The long-term sequelae of this type of injury include mental retardation, spastic quadriparesis, and seizures. Choreoathetosis and dystonia may be seen if the thalamus and basal ganglia are involved. Of note, the basal ganglia seem to be particularly sensitive to hypoxia. Bulbar and pseudobulbar palsy can occur if the brainstem and tegmentum are affected. Hypoperfusion with subsequent reperfusion injury and glutamate-induced injury are involved in the pathogenesis of SNN. The four major patterns of injury include (1) diffuse, (2) cerebral cortex–deep gray matter, (3) deep gray matter–brainstem, and (4) pontosubicular injury.
Diffuse neuronal injury
This type of SNN occurs after severe, very prolonged hypoxic–ischemic insults in both term and premature infants and affects nearly all neurons in the neuroaxis. It most frequently affects the cortex, hippocampus, cerebellum, and anterior horn cells of the spinal cord. Within the cortex, the injury is more prominent in the watershed zones and more marked in the depth of the sulci than in the gyri. With more severe injuries, the more differentiated visual (calcarine) cortex and the perirolandic cortex may be damaged.
Cerebral cortex–deep gray matter
This type of SNN occurs mainly in term neonates after moderate to severe, usually prolonged hypoxic–ischemic insults. Injury is generally bilateral and typically affects the dorsolateral putamen and ventrolateral thalami. This combination is typical of HII in the term neonate.
Deep gray matter–brainstem
This type of SNN occurs mainly in term neonates after severe, prolonged insults that are fairly abrupt in onset. Although injury to the basal ganglia and thalamus occurs in roughly 75% of these patients, only 15% to 20% of neonates with HII exhibit involvement of the deep gray matter and brainstem as the prevailing lesion that is present, and only a portion of these evolve to “status marmoratus.” Status marmoratus is so-named because of the marbled appearance acquired by the affected structures (particularly the putamen) after this type of injury. It is characterized by neuronal loss, gliosis, and hypermyelination. The complete pathologic picture is not apparent until at least 8 months after birth, although the insult occurs in the perinatal period. The clinical condition manifests later in life with cognitive deficits and movement disorders, including choreoathetosis and dystonia, which may not be apparent until 1 to 4 years of age.
Pontosubicular injury
This type of SNN affects the neurons of the basis pontis and the subiculum of the hippocampus. This type of injury occurs mainly in preterm neonates and is strongly associated with PVL. Clinically, it is associated with hypoxia–ischemia, hypocarbia, and hyperoxia.
Focal or Multifocal Ischemic Brain Lesions
This form of injury is unusual before the 28th week of gestation. Its incidence increases with gestational age and most commonly occurs as a result of postnatal events. It mainly affects the middle cerebral artery territory, and it affects the left middle cerebral artery more often than it affects the right. When venous structures are affected, superior sagittal sinus thrombosis is the most common causative lesion. The major causes of these lesions include perinatal asphyxia, infection, trauma, and coagulation disorders. However, the etiology of this type of injury remains unknown in roughly half of patients.
Differentiation between these various types of brain lesions, especially between focal or multifocal necrosis, parasagittal injury, and PVL, can be challenging, and in many cases these patterns of injury coexist.
At the molecular level, it has recently been postulated that HII occurs in two steps in the mature fetal brain: ischemia and reperfusion. The first is believed to be the result of an acute reduction in umbilical or uterine circulation. Recently, it has been postulated that a considerable proportion of neuronal injury occurs during the second phase of neuronal cell damage, the reperfusion phase. This is believed to result from several different types of inflammatory reactions in conjunction with the inhibition of protein synthesis, which eventually leads to the induction of neuronal apoptosis (programmed cell death). There are also increasing data showing an association between HII and ascending intrauterine infections either before or after birth, possibly caused by endotoxin-mediated cytokine release. Furthermore, it is believed to be a synergistic effect between infection and HII.
US characteristics of NE
The classic imaging findings of acute HII have been widely described and typically include focally or diffusely increased echogenicity of the brain parenchyma and a slit-like appearance of the ventricles with obliteration of the extracerebral cerebral spinal fluid spaces and the interhemispheric fissure. However, in the authors’ experience, these findings are usually seen only in advanced cases of NE. In less severe cases, the findings are less conspicuous and can only be appreciated when linear or magnified US images are obtained. This is not a novel or exotic finding, because as far back as 1994, Eken and colleagues reported on the improved use of high-resolution US images obtained with high MHz transducers compared with conventional US images. They compared US findings obtained with high MHz probes with autopsy findings in 20 patients using a high-resolution 10-MHz transducer to better depict pathology in several locations in the brain. They found that this technique was particularly useful for identifying lesions in the thalamus, and that it was able to do so with a sensitivity of 100% and a specificity of 83%. This technique was also excellent at depicting lesions in other regions of the brain. The authors found a sensitivity and specificity of 100% and 83% for cortical lesions and 77% and 100% for white matter lesions, respectively.
This article is based on a review of the existing medical literature and the authors’ 29-month experience with 76 neonatal patients in whom brain US and MR imaging were prospectively obtained within 2 hours of one another. This cohort of patients forms the basis for the current discussion, with an emphasis placed on subtle, lesser known findings that may improve the diagnosis and characterization of NE. The main purposes of this article are to increase readers’ awareness of the subtle findings on linear or magnified US images (which are usually more conspicuous on MR imaging and may be associated with the presence of severe disease) that may allow a diagnosis of NE to be made; and to emphasize the technical factors that may optimize US imaging of these patients. It is well beyond the scope of this article, however, to review the detailed management of NE or to describe in detail the MR imaging findings that can be observed in patients with this condition.
In the authors’ experience, the head US findings associated with NE may be divided into three main groups to allow for better characterization of the lesions that are present:
- 1.
Peripheral brain
- a.
Gray–white matter differentiation
- b.
Cortical abnormalities
- c.
Subcortical white matter
- a.
- 2.
Central findings
- a.
Basal ganglia
- b.
Brainstem and posterior fossa
- c.
Periventricular white matter
- d.
Ventricular size
- a.
- 3.
Doppler findings
- a.
Resistive indices
- b.
Hyperemia
- c.
Sinus vein patency
- a.
Peripheral US findings
Gray–white Matter Differentiation
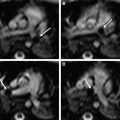
Differentiation between gray and white matter is possible with the use of high-frequency linear array transducers. The sulci demonstrate moderately high echogenicity as opposed to the gyri. Both gray and white matter are relatively hypoechoic and homogeneous in appearance, and the cortical gray matter is only slightly more hypoechoic than the adjacent subcortical white matter. Thus, the boundary between gray and white matter is subtle within the normal brain ( Fig. 1 ).
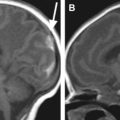
Accentuation of gray–white matter differentiation (GWMD) is an early sign of brain edema ( Fig. 2 ). As edema worsens, the classically described appearance of slit-like ventricles, sulcal effacement, and either generalized or patchy increases in the echogenicity of the brain parenchyma is seen. In advanced cases GWMD is lost ( Fig. 3 ).
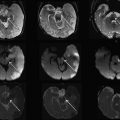
The differential diagnosis for this pattern of accentuation of GWMD includes congenital infections and leukodystrophies, such as Canavan disease and Alexander disease ( Fig. 4 ).