Figure 9.2.1
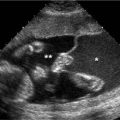
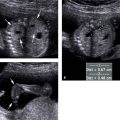
Hypoplastic left ventricle. Transverse images of fetal thorax at level of four-chamber view of the heart in fetus with hypoplastic left ventricle, demonstrating small left ventricle (LV arrow) and larger right ventricle (RV arrow) (S = spine, 4CH = four-chamber heart).
With ventricular dilatation and endocardial fibroelastosis as a result of severe aortic stenosis or aortic atresia, the left ventricle may initially become enlarged and globular, with increased echogenicity along the inner wall and poor ventricular contractility (Figure 9.2.3). As pregnancy progresses, the dilated, globular left ventricle may become progressively smaller in utero until it becomes hypoplastic. The increased echogenicity of the ventricular wall will persist (Figure 9.2.4).
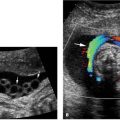
Aortic stenosis is characterized by narrowing of the aortic valve and decreased movement of the valve leaflets. The narrowed aortic valve can be visualized on a long axis view of the left ventricle and left ventricular outflow tract (Figure 9.2.5). The width of the valve can be measured and compared with norms for gestational age. The stenotic valve is often brightly echogenic. The ascending aorta may be enlarged due to poststenotic dilation. Color Doppler will demonstrate a narrow, high-velocity jet of flow across the stenotic valve (Figure 9.2.6), instead of the normal broad jet of flow.
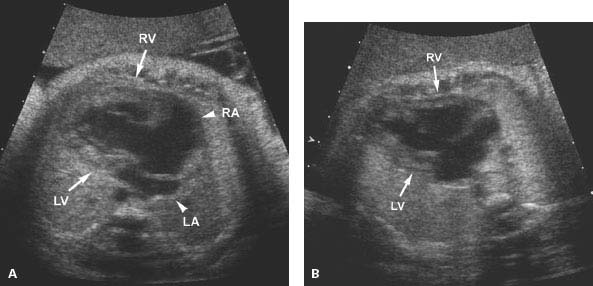
Figure 9.2.2
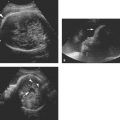
Hypoplastic left ventricle with no left ventricular chamber. A: Transverse image of thorax demonstrating heart with large right ventricle (RV arrow) and no appreciable left ventricular chamber (LV arrow) (LA arrow = left atrium, RA arrow = right atrium). B: Transverse image of heart in another fetus with large right ventricle (RV arrow) and no visible left ventricular chamber (LV arrow).
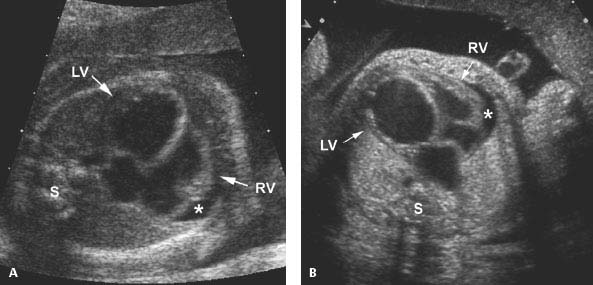
Figure 9.2.3
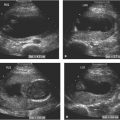
Dilated left ventricle with endocardial fibroelastosis, developing into hypoplastic left heart. (A) and (B) Four-chamber views of the heart in two fetuses, demonstrating echogenic left ventricular walls (LV arrow) with poor left ventricular contractility. A small pericardial effusion (*) is present in each (RV arrow = right ventricle, S = spine). Both of these fetuses went on to develop hypoplastic left ventricles.
Mitral regurgitation commonly accompanies aortic stenosis when hypoplastic left heart is developing. With color Doppler imaging the regurgitation appears as retrograde flow across the mitral valve during cardiac systole (Figure 9.2.6).
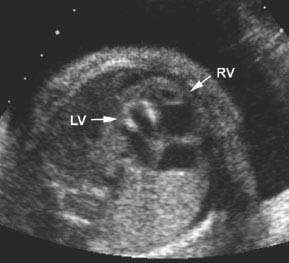
Figure 9.2.4
Hypoplastic left ventricle with echogenic walls. Four-chamber view of heart showing very small left ventricle (LV arrow) with brightly echogenic walls and compensatorily enlarged right ventricle (RV arrow).
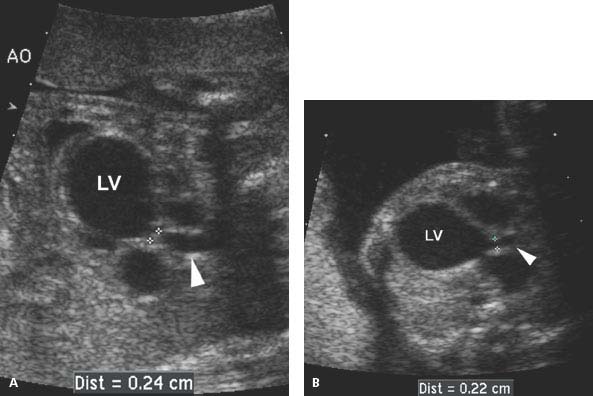
Figure 9.2.5
Aortic stenosis with dilated left ventricle. (A) and (B) Longitudinal images of left ventricular (LV) outflow tract to aorta (arrowhead) in two fetuses with aortic stenosis. The aortic valve (calipers) is narrowed and the left ventricle (LV) is dilated in each.
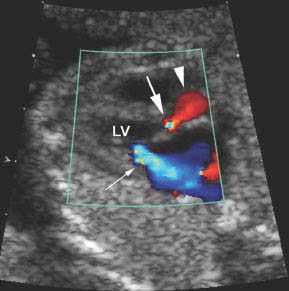
Figure 9.2.6
Aortic stenosis and mitral regurgitation with color Doppler. Oblique image of heart showing left ventricular (LV) outflow tract with color Doppler, demonstrating narrowing of the color jet (large arrow) with aliasing of the color signal, indicating high velocity across the stenotic valve. The ascending aorta is dilated (arrowhead) due to poststenotic dilation above the valve. Also seen is a large jet of retrograde flow across the mitral valve (small arrow), representing mitral regurgitation, which often accompanies critical aortic stenosis.
9.3. Hypoplastic Right Ventricle and Pulmonic Stenosis
Description and Clinical Features
A hypoplastic right ventricle, which is less common than hypoplastic left heart, is characterized by a small or absent right cardiac ventricle. This most often results from pulmonic stenosis or atresia with an intact ventricular septum, but can also result from stenosis or atresia of the tricuspid valve. With any of these, there is obstruction of blood flow either into the right ventricle or out of the right ventricle, leading to shunting of blood across the foramen ovale to the left side of the heart. The left ventricle may be enlarged and hypertrophic. A hypoplastic right ventricle may cause fetal cardiac failure and hydrops.
Pulmonic stenosis is characterized by an abnormal pulmonic valve that obstructs the blood flow through the right ventricular outflow tract. The stenosis may be an isolated abnormality of the heart or a component of a more complex congenital cardiac malformation such as tetralogy of Fallot or Uhl anomaly. With isolated pulmonic stenosis, the right ventricle may be small or large, depending on the degree of shunting of blood across the foramen ovale and the extent of tricuspid regurgitation.
Sonography
A hypoplastic right ventricle is best diagnosed on a four-chamber view of the heart when the right ventricle is smaller than the left ventricle (Figure 9.3.1). Often the small right ventricle has thickened walls, sometimes markedly (Figure 9.3.2), and ventricular contractility is usually poor. In rare cases, no right ventricle can be found (Figure 9.3.3). In the latter case, the distinction between hypoplastic right ventricle and hypoplastic left ventricle may be difficult.
With pulmonic stenosis, there is narrowing at the level of the pulmonic valve (Figure 9.3.4). Measurement of the pulmonic valve can be compared with norms for gestational age to assess the degree of narrowing. Poststenotic dilation of the pulmonic artery may be seen in some cases of isolated pulmonic stenosis (Figure 9.3.5). With pulmonic atresia or critical pulmonic stenosis, retrograde flow may be seen in the ductus arteriosus, carrying blood from the aorta to the pulmonary arteries. The reversed flow in the ductus arteriosus is best seen on a transverse color Doppler image of both the ductal arch and the aortic arch, and is diagnosed when flow in the ductal arch is in the opposite direction to flow in the aortic arch (Figure 9.3.6). Careful assessment for accompanying cardiac anomalies is warranted, especially looking for abnormalities of the tricuspid valve and ventricular septum.
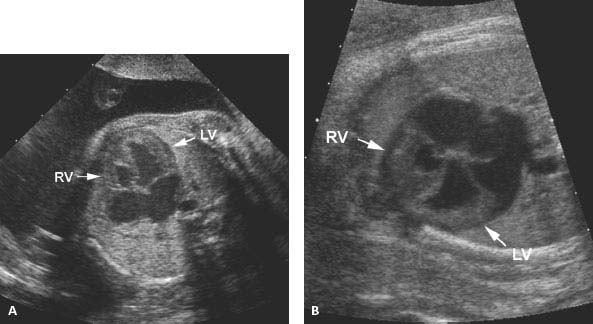
Figure 9.3.1
Hypoplastic right ventricle. (A) and (B) Transverse image of fetal thorax at level of four-chamber view of the heart in two fetuses, demonstrating a small right ventricle (RV arrow) and a larger left ventricle (LV arrow) in each.
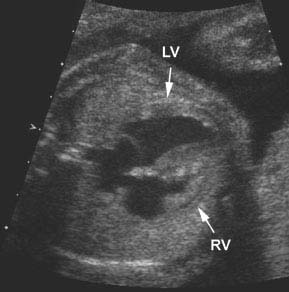
Figure 9.3.2
Hypoplastic right ventricle with marked thickening of the ventricular walls and poor contractility. Axial image of chest at level of four-chamber view of the heart showing a small right ventricle (RV arrow) with markedly thickened walls, including the septum (LV arrow = left ventricle).
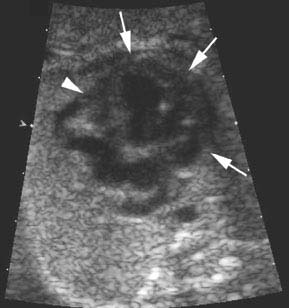
Figure 9.3.3
Hypoplastic right ventricle with no right ventricular chamber. Four-chamber view of the heart demonstrating a large left ventricle (arrows) and no appreciable right ventricle (arrowhead).
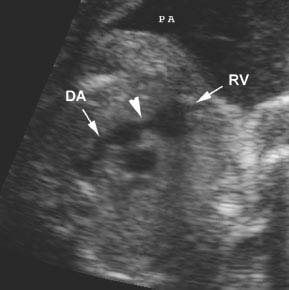
Figure 9.3.4
Pulmonic stenosis. Axial view of right ventricular outflow tract demonstrating narrowing at the level of the pulmonic valve (arrowhead) between the right ventricle (RV arrow) and main pulmonary artery to the ductus arteriosus (DA arrow).

Figure 9.3.5
Pulmonic stenosis with poststenotic dilatation of the pulmonary artery. Oblique image of right ventricular (RV arrow) outflow tract demonstrating narrow pulmonic valve (calipers) with poststenotic dilatation of the pulmonary artery (PA) (arrows) (post = posterior).
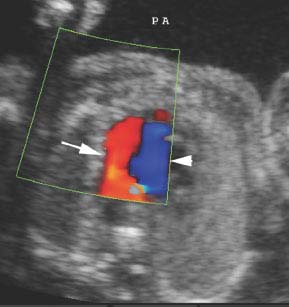
Figure 9.3.6
Severe pulmonic stenosis with reversed flow in the ductus arteriosus. Transverse view of the fetal chest at the level of the ductal arch (arrow) and aortic arch (arrowhead) showing reversed flow in the ductal arch, from the aorta to the main pulmonary artery (PA). Normal antegrade flow is seen in the aortic arch.
9.4. Ebstein Anomaly
Description and Clinical Features
Ebstein anomaly is an anomaly that results from the malformation and malposition of the tricuspid valve. The valve is displaced into the right ventricle and is dysplastic and incompetent, leading to tricuspid regurgitation and enlargement of the right atrium. During atrial systole, blood flows from the right atrium toward the apex of the right ventricle. During ventricular systole, the blood regurgitates from the portion of the right ventricle distal to the tricuspid valve back across the dysplastic tricuspid valve into the right atrium. The right atrium can become markedly enlarged. Hydrops may develop in utero due to fetal cardiac failure.
The prognosis for this cardiac anomaly, when diagnosed prenatally is poor, with a mortality of 35–50%. Some fetuses die before birth and others die in the neonatal period. The prognosis is particularly poor if hydrops develops in utero or when there is pulmonary hypoplasia as a result of compression of the lungs by the enlarged heart. Long-term survivors of Ebstein anomaly often have persistent cardiac arrhythmias.
Sonography
With Ebstein anomaly, the four-chamber view of the heart is abnormal. The heart is markedly enlarged, especially the right atrium, and the tricuspid valve is displaced toward the apex of the right ventricle (Figure 9.4.1). Tricuspid regurgitation can be demonstrated with color or spectral Doppler (Figure 9.4.2).
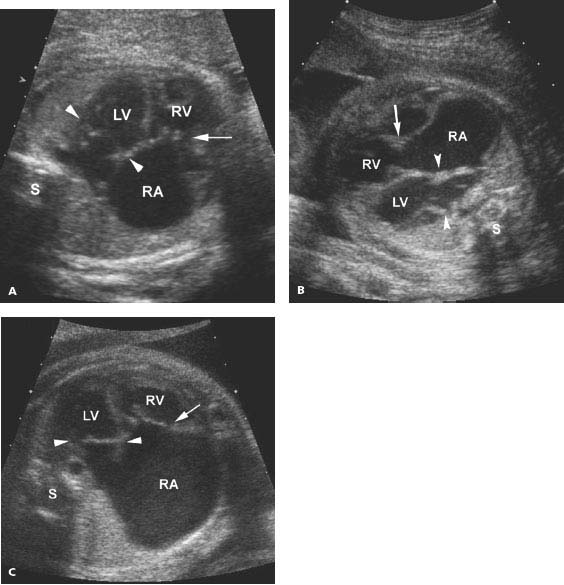
Figure 9.4.1
Ebstein anomaly. (A), (B), and (C) Transverse images of thorax, with four-chamber views of heart, demonstrating (A) and (B) moderately, and (C) markedly enlarged right atrium (RA) and mildly enlarged right and left ventricles (RV and LV). The tricuspid valve (arrow) is displaced into the right ventricle below the level of the normally positioned mitral valve (arrowheads) (S = spine).
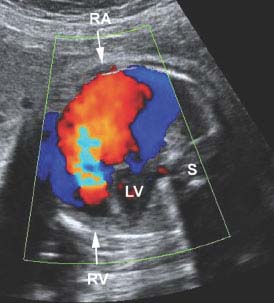
Figure 9.4.2
Color Doppler of Ebstein anomaly with tricuspid regurgitation. Color Doppler images of four-chamber view of heart demonstrating a large jet of retrograde flow (red with central aliasing) across the abnormal triscuspid valve from the right ventricle (RV arrow) back into right atrium (RA arrow) during ventricular systole (LV = left ventricle, S = spine).
9.5. Ventricular Septal Defect
Description and Clinical Features
An opening in the muscular or membranous portion of the interventricular septum is called a ventricular septal defect. These defects may be small and clinically insignificant or quite large, causing significant shunting of blood across the defect. Some of the small defects close spontaneously after birth. Defects in the membranous portion of the septum are more common than those in the muscular septum and tend to be smaller. Prenatally, blood flow through the septal defect is typically from the right to the left ventricle. After birth, shunting across the defect changes to be from left to right due to changes in pressure in the cardiac ventricles.
Ventricular septal defects may be isolated anomalies or part of a more complex cardiac malformation. Isolated defects have an excellent prognosis.
Sonography
The diagnosis of a ventricular septal defect is made when a gap is seen in the septum between the right and left ventricles. Many ventricular septal defects can be diagnosed on a four-chamber view of the fetal heart (Figure 9.5.1). Other defects will only be seen on a long axis view of the left ventricle and left ventricular outflow tract (Figure 9.5.2). On the latter view, smaller defects in the membranous portion of the septum, not visible on the four-chamber view, may be seen. Some ventricular septal defects, especially small membranous ones, may not be visible at all on prenatal ultrasound.
Flow across ventricular septal defects in utero, typically from the right to the left ventricle, can be seen with color Doppler imaging (Figure 9.5.3).
Because a ventricular septal defect may be a component of a complex cardiac anomaly, careful assessment of ventricular and atrial chamber sizes, atrioventricular valves, ventricular outflow tracts, and the atrial septum is warranted.
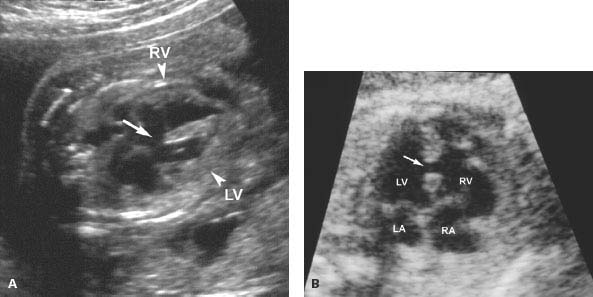
Figure 9.5.1
Ventricular septal defects. A: Four-chamber image of heart demonstrating large defect (arrow) in the muscular septum between the right and left ventricles (RV arrowhead and LV arrowhead).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree