and Urs Ziegler1
(1)
Center for Microscopy and Image Analysis, University of Zurich, Zurich, Switzerland
Abstract
In this chapter we discuss the latest developments in the field of high-pressure freezing (HPF). The Leica HPF machine EM HPM100 is discussed in detail due to significant changes compared to its predecessor model. Its centerpiece is a multipart polymer cartridge which holds the specimen carrier sandwich and guides it automatically through the freezing process until immersed in liquid nitrogen. The cartridge can be adapted to the specimen and carrier geometry to optimize the flow of liquid nitrogen and hence rapid cooling. Dedicated cartridges are available for a variety of different carriers, including carriers for samples of up to 5 mm in diameter. Cartridge-specific handling and carrier assemblies are described extensively for freezing samples in aluminum specimen carriers, cell cultures grown on Sapphire discs, suspensions for freeze-fracturing, and specimens for cryo-sectioning. Additionally, we include an advanced technique to freeze monolayer cell cultures on Sapphire discs with the Leica EM PACT2 HPF machine using a composite carrier.
Key words
High-pressure freezingFreeze-substitutionFreeze-fracturingCryo-sectioningMonolayer cell culturesSapphire disc1 Introduction
Cryo-immobilization is the optimum technique to preserve the ultrastructure of biological specimens or more generally, soft condensed matter. However, freezing of samples thicker than a few microns without the use of antifreezing agents and at ambient pressure leads to considerable ice crystal formation and distortion of the biological material. Such samples can only be frozen adequately if intracellular antifreezing agents are used or under high pressure. Intracellular antifreezing agents are normally not incorporated by living cells or organisms under physiological conditions or are problematic concerning normal physiology of the cell. High-pressure freezing (HPF) was developed to overcome these drawbacks and allows adequate freezing of native biological samples of up to 200 μm in thickness. The optimum pressure of 2,100 bar needs to be applied to the sample within a few milliseconds followed by immediate rapid cooling to prevent the introduction of artifacts caused by high pressure (e.g., changes of dissociation constants). HPF became a state-of-the-art technique to contribute to the elucidation of numerous scientific questions which cannot be answered using room temperature preparation techniques like chemical fixation.
Currently, four different HPF machines are available: EM HPM100 and EM PACT2 (Leica Microsystems, Vienna, Austria), HPM 010 (Boeckeler Instruments, Tucson, AZ, USA), and HPF Compact 02 (Engineering Office M. Wohlwend, Sennwald, Switzerland). EM HPM100, HPM 010, and HPF Compact 02 are systems using liquid nitrogen at high pressure to pressurize and to cool the specimen in a small container located in the pressure chamber. A small volume of alcohol at room temperature is usually introduced first to coordinate pressure buildup and cooling. In the EM PACT2 system, the specimen in the container is pressurized with a transmission fluid (methylcyclohexane) and subsequently cooled with a stream of liquid nitrogen at 10 bars [1–3].
Several articles about HPF have been published in the last years. In particular, they focus on specimen preparation and handling before freezing, which are the most important steps to make HPF a successful method to preserve the ultrastructure of biological specimens [4–16]. Specimen processing after freezing is also extensively described in the literature for a variety of different specimens and applications. This chapter will focus on the recent developments regarding HPF machines, specimen carriers and methods rather than providing a review of previous articles. The recently developed HPF machine EM HPM100 is described in detail. Handling and specimen preparation is discussed as far as different from the other systems (HPM 010 and HPF Compact 02). Additionally, an improved method for freezing cell culture monolayers grown on Sapphire discs with the EM PACT2 system is presented.
The EM HPM100 is a largely automated system with an integrated workstation and binocular microscope (see Fig. 1a). The centerpiece is a versatile cartridge, consisting of highly durable polymer pieces (see Fig. 1b). The assembled cartridge holds the specimen carrier sandwich (or other container) and guides it automatically through the freezing process, finally releasing cartridge, carriers, and sample in liquid nitrogen. This guarantees that the whole freezing sequence from pickup of the specimen to liquid nitrogen is consistent and reproducible.


Fig. 1
(a) High-pressure freezing (HPF) machine EM HPM100. Front view showing workstation, binocular microscope and touch screen. (b) Exploded view of the cartridge system of the EM HPM100 for 3 mm specimen carriers: Identical upper and lower half cylinders and a middle plate with bore for the specimen carrier sandwich. Arrows indicate the flow of liquid nitrogen during the cooling process. Images courtesy of Leica Microsystems
A wide range of different specimen geometries can be frozen with the according set of cartridge pieces and specimen carriers. In particular, samples of up to 5 mm in diameter can be processed, which facilitates handling before freezing considerably. The cartridge is made of a thermally insulating polymer leading to cooling of the sample and carriers rather than the surrounding cartridge and to optimized heat extraction from the sample. Additionally, the volume of the pressure chamber was reduced compared to the previous HPM 010 model resulting in a quicker pressure buildup, higher flow rate of liquid nitrogen, and less nitrogen consumption.
Injection of alcohol at room temperature into the pressure chamber to coordinate pressure buildup and cooling can be switched off on demand. Application of this option will be discussed later in this chapter. A chamber to keep the temperature and humidity at desired values is available for the work station. The EM HPM100 is mobile and the remaining liquid nitrogen in the storage Dewar and pressure cylinder can be retrieved after a freezing session similar to the EM PACT2. Temperature and pressure courses are recorded and can be stored. The freezing quality and yield of well frozen samples are comparable to the results achieved with HPM 010 [6, 17–21].
The option of freezing specimens of a diameter up to 5 mm is appreciated by many scientists in the field and lead recently to adaptations also of HPM 010 and HPF Compact 02. Appropriate holders for up to 6 mm specimen carriers are now available for HPM 010 (per. comm. Bill Graham, Bibst Labs, Brookline, NH) and HPF Compact 02. An additional new feature of the HPF Compact 02 is that specimen holder containing the specimen carrier sandwich is now introduced horizontally into the pressure chamber compared to the vertical insertion with the predecessor model (per. comm. M. Wohlwend, Engineering Office M. Wohlwend, NH).
2 Materials
2.1 High-Pressure Freezing Machines
1.
EM HPM100 with essential accessories (specimen carrier release device).
2.
EM PACT2 with essential accessories (bayonet pod, loading station, rapid transfer system loading device, transfer slider, torque wrench).
Cartridges, specimen carriers and HPF machine associated parts are available from Leica Microsystems, else indicated. A wide variety of specimen carriers for all types of HPF machines are also available from Engineering Office M. Wohlwend.
2.2 EM HPM100 Specimen Carriers and Cartridges
2.2.1 3 mm Aluminum Specimen Carrier System
1.
3 mm cartridge (half cylinders and middle plate).
2.
3 mm aluminum specimen carrier Type A (100 μm/200 μm recess).
3.
3 mm aluminum specimen carrier Type B (flat/300 μm recess).
4.
3 mm aluminum specimen carrier Type C (slit carrier, 100 μm/200 μm recesses).
5.
3 mm aluminum specimen carrier with 150/150, 275/25, or 250/50 μm recess (Engineering Office M. Wohlwend).
6.
3 mm punch for specimen carrier release device.
7.
Round file to widen bore of the middle plate.
2.2.2 6 mm Aluminum Specimen Carrier System
1.
6 mm cartridges (half cylinders and middle plate).
2.
6 mm aluminum specimen carrier Type A (100 μm/200 μm recess).
3.
6 mm aluminum specimen carrier Type B (flat/300 μm recess).
4.
6 mm aluminum specimen carrier with 150/150 μm recesses (Engineering Office M. Wohlwend).
5.
6 mm punch for specimen carrier release device.
2.2.3 Cell Monolayers on 3 mm Sapphire Discs
1.
3 mm Sapphire discs.
2.
6 mm cartridge half cylinders.
3.
6 mm cartridge middle plate with edge.
4.
6 mm support rings for 3 mm Sapphire discs.
5.
3 mm aluminum specimen carrier Type B (flat/300 μm recess).
6.
Cell culture dishes with optically appropriate glass or plastic bottom (MatTek Corporation, Ashland, MA, USA and ibidi GmbH, Planegg/Martinsried, Germany).
2.2.4 Cell Monolayers on 6 mm Sapphire Discs
1.
6 mm Sapphire discs.
2.
6 mm cartridge half cylinders.
3.
6 mm cartridge middle plate with edge.
4.
6 mm aluminum specimen carrier Type A (100 μm/200 μm recesses).
5.
6 mm spacer ring 200 μm thickness.
6.
6 mm finder grid mask.
7.
Cell culture dishes with optically appropriate glass or plastic bottom (MatTek Corporation and ibidi GmbH).
2.2.5 Freeze-Fracturing of Thin Suspensions
1.
3 mm cartridges (half cylinders and middle plate).
2.
3 mm aluminum specimen carrier Type B (flat/300 μm recess).
3.
Scalpel.
4.
TEM grids of choice.
5.
Round file to widen bore of the middle plate.
2.2.6 Cryo-sectioning Using Copper Specimen Tubes
1.
Cartridges for tubes (half cylinders).
2.
Copper specimen tubes (outer diameter 0.65 mm, inner diameter 0.35 mm, length 9.5 mm).
3.
Tube press.
4.
Tube holder for cryo-ultramicrotome.
2.2.7 Cryo-sectioning Using Freeze-Fracture and Membrane Carrier
1.
3 mm cartridges (half cylinders and middle plate).
2.
Gold hat freeze-fracture carrier 3 mm with cylindrical recess, thickness 0.8 mm.
3.
Membrane carrier 2.8 mm diameter with recess of 100 or 200 μm.
4.
Cryo-ultramicrotome holder for gold hat carrier (not yet available commercially) or cryo-glue and aluminum stubs.
2.3 EM PACT2 Carrier Systems
2.3.1 Cell Monolayers on 1.4 mm Sapphire Discs
1.
Composite carrier consisting of gold ring and 1.4 mm Sapphire disc (available from Engineering Office M. Wohlwend).
2.4 Specimen Preparation and Processing
1.
Scalpel.
2.
Razor blades.
3.
Forceps, fine and blunt.
4.
Tape.
5.
Plastic Petri dishes.
6.
1.5 and 2 ml Eppendorf tubes.
7.
Diamond marking pen.
8.
Millicell membrane inserts (0.4 μm, Millipore, EMD Millipore Corporation, Billerica, MA, USA).
9.
Vibratome.
10.
Biopsy punches (4 or 5 mm).
11.
Cellulose capillary tubes (Leica Microsystems).
12.
1-Hexadecene (e.g., 92 %, Thermo Fisher Scientific, Waltham, MA, USA).
13.
Yeast paste (Baker’s yeast as thick suspension).
14.
Dextran (MW 39,000, Sigma-Aldrich, St. Louis, MO, USA) solution 20 % in an appropriate buffer for the sample.
15.
Polylysine (Sigma-Aldrich).
3 Methods
For optimal results, the following basic rules for HPF are essential: Always use a fresh sample and as thin as possible, preferably thinner than 200 μm. Remove extracellular medium containing high amounts of water and substitute with a filler (e.g., 1-hexadecene, yeast paste, Dextran solution). Make sure that no air is trapped in the specimen carriers.
3.1 Loading of Samples and Handling Using the EM HPM100
Procedures for sample preparation as well as all specimen carrier sandwich configurations used with the HPF machines HPM 010 and HPF Compact 02 also apply to the EM HPM100. Cartridges are available for all specimen carriers used routinely with HPM 010 and HPF Compact 02 systems.
3.1.1 Freezing Using the 3 and 6 mm Aluminum Specimen Carrier System
1.
Place the three components of the cartridge for the 3 or 6 mm carrier system (upper, lower half cylinders and middle plate) in the correct position at the work station as shown in Fig. 2a, b. Upper and lower half cylinders are identical.
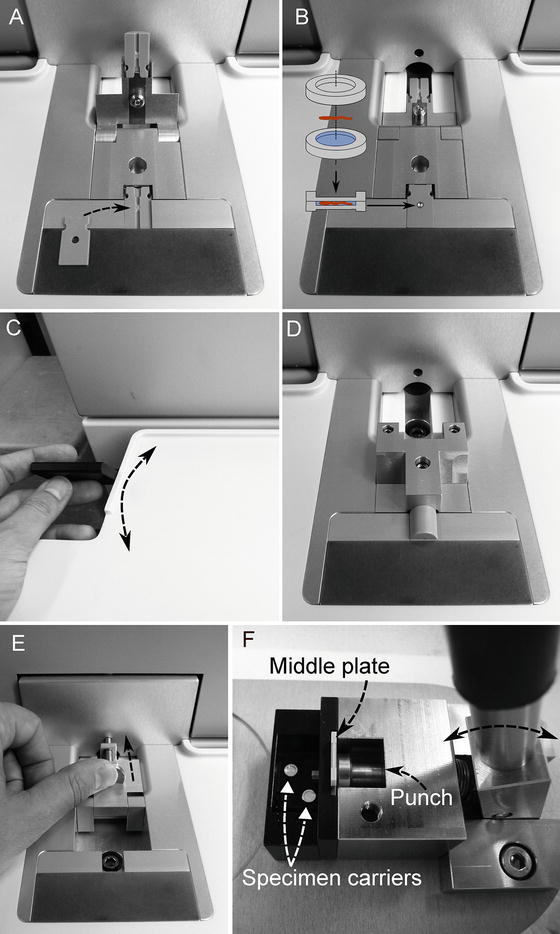

Fig. 2
Operations required for freezing samples with the EM HPM100. (a) Placing of the cartridge half cylinders at the work station. The arrow indicates the positioning of the middle plate. (b) 3 mm specimen carrier sandwich inserted into the recess of the positioned middle plate. (c) The handle on left side of the workstation is flipped to pick up the specimen and to complete the cartridge as depicted in (d). (e) Subsequently, the handle is flipped back and the process is started by pressing the safety button. After freezing, the cartridge with the frozen specimen is automatically transferred into the liquid nitrogen container in the drawer on the left side of the machine. (f) The specimen carrier sandwich is released from the middle plate by using the specimen carrier release device, which acts as a punch
2.
3.
4.
Flip the handle on the left side of the work station to complete the cartridge assembly containing carrier sandwich with specimen (see Fig. 2c, d).
5.
Flip the handle backwards and press the safety lock at the front of the machine to start the freezing process (see Fig. 2e).
6.
Remove the cartridge pieces and the specimen carriers from the liquid nitrogen Dewar in the drawer on the left side of the machine.
The 6 mm aluminum specimen carrier system offers freezing of samples up to 5 mm in diameter. In most cases, only a small part of such a large sample will be needed for imaging in the electron microscope. However, the provided space for the specimen noticeably facilitates handling and loading of the sample before freezing in many cases (see following paragraphs).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree