Hyperparathyroidism
Pathophysiology
Hyperparathyroidism, also known as generalized osteitis fibrosa cystica or Recklinghausen disease of bone, is the result of overactivity of the parathormone-producing parathyroid glands. Increased production of this hormone is secondary to either gland hyperplasia (9% of cases) or adenoma (90%); only in very rare instances (1%) does hyperparathyroidism occur secondary to parathyroid carcinoma. Excessive secretion of parathormone, which acts on the kidneys and on bone, leads to disturbances in calcium and phosphorus metabolism, resulting in hypercalcemia, hyperphosphaturia, and hypophosphatemia. Renal excretion of calcium and phosphate is increased, and serum levels of calcium are elevated, while those of phosphorus are reduced. Serum levels of alkaline phosphatase are also elevated.
Hyperparathyroidism can be divided into primary, secondary, and tertiary forms. The classic form of the disorder, primary hyperparathyroidism, is marked by an increased secretion of parathormone resulting from hyperplasia, adenoma, or carcinoma of the parathyroid glands. Primary hyperparathyroidism is usually associated with hypercalcemia. Women are affected about three times as often as men, and the condition is most commonly seen in the patient’s third to fifth decade. Primary hyperparathyroidism is a genetically heterogenous disorder caused by mutations in the MEN1, CDC73, or CASR gene. The MEN1 gene regulates production of a protein menin, which acts as a tumor suppressor. The CDC73 gene provides instructions for making the parafibromin protein, another tumor suppressor. The loss of parafibromin’s tumor suppressor function leads to the development of parathyroid adenoma, or parathyroid carcinoma. The CASR gene provides instructions for producing a protein called the calcium-sensing receptor (CaSR) responsible for regulating the amount of calcium in the body, in part by controlling the production of parathormone.
Secondary hyperparathyroidism is caused by an increased secretion of parathyroid hormone (PTH) in response to a sustained hypocalcemic state. Usually, the fundamental cause of parathyroid gland hyperfunction is impaired renal function. Hyperphosphatemia due to renal failure results in chronic hypocalcemia, which in turn promotes increased parathyroid secretion. Although secondary hyperparathyroidism is usually hypocalcemic, it may be normocalcemic as an adaptive response to the hypocalcemic state. Tertiary hyperparathyroidism represents a transformation from a hypocalcemic to a hypercalcemic state. The parathyroid glands “escape” from the regulatory effect of serum calcium levels. Patients in whom this escape occurs are usually receiving kidney hemodialysis; they are considered to have autonomous hyperparathyroidism.
Although primary hyperparathyroidism is traditionally synonymous with the hypercalcemic form of the disorder, some patients nonetheless may have normal or even reduced serum calcium levels. For this reason, Reiss and Canterbury proposed an alternative method of classifying hyperparathyroidism based on serum calcium levels. In this system, hyperparathyroidism is considered either hypercalcemic, normocalcemic, or hypocalcemic.
In order to understand the clinical, pathologic, and imaging manifestations of hyperparathyroidism, knowledge of the interrelated roles of PTH and vitamin D in the metabolism of calcium is essential.
Physiology of Calcium Metabolism
Serum concentrations of calcium are maintained within a narrow normal physiologic range (2.20 to 2.65 mmol/L or 8.8 to 10.6 mg/dL) by the intestines and kidneys, the major sites of classic negative feedback mechanisms that balance calcium intake and excretion. The bones also contribute to preserving calcium homeostasis and, since they represent approximately 99% of elemental calcium in the human body, are considered to be a calcium reservoir. Essential to these mechanisms involving a variety of hormones is the action of PTH, a polypeptide hormone whose secretion is induced by a decrease in the level of calcium in the extracellular fluid. In primary hyperparathyroidism, there is inappropriate oversecretion of PTH in the presence of elevated serum calcium levels, while secondary hyperparathyroidism is marked by appropriate PTH production in response to chronic hypocalcemia.
PTH works to increase serum calcium concentrations by several means. Predominant among these is conserving calcium in the kidneys by promoting both increased reabsorption of calcium and increased excretion of phosphates in the distal renal tubules. PTH also promotes release of calcium and phosphorus from bone by increasing the number and activity of osteoclasts, resulting in bone resorption, although the exact mechanism by which this occurs is not fully understood. Finally, although PTH has been shown to have no direct effect on intestinal calcium absorption, it plays a role in stimulating vitamin D metabolism, with subsequent increased absorption of calcium and phosphorus by the intestines.
Both forms of vitamin D in the human body—ergocalciferol (vitamin D2), a synthetic compound and frequent food additive; and cholecalciferol (vitamin D3), formed predominantly in the skin from 7-dehydrocholesterol by the action of ultraviolet light—are metabolized to 25-hydroxyvitamin D in the liver. The critical step in the metabolism of vitamin D occurs in the kidneys, where 25-hydroxyvitamin D undergoes hydroxylation to its most active form, 1,25-dihydroxyvitamin D, and an inactive metabolite, 24,25-dihydroxyvitamin D. This step is catalyzed by the renal enzyme 1-α-hydroxylase, which is synthesized in the kidneys under the stimulation of PTH in the presence of decreased serum calcium and phosphate levels. This gives the kidneys a unique central role in the metabolism of vitamin D. 1,25-Dihydroxyvitamin D is the primary mediator of calcium and phosphorus absorption in the small intestine. The kidneys also have the ability to switch between producing the active and inactive forms of vitamin D, yielding a fine control of calcium metabolism.
The symptoms of hyperparathyroidism are related to hypercalcemia, skeletal abnormalities, and renal disease. Hypercalcemia produces weakness, muscular hypotonia, nausea, anorexia, constipation, polyuria, and
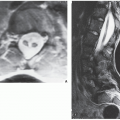
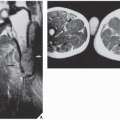
thirst. The skeletal abnormalities most commonly seen are generalized osteopenia and foci of bone destruction, which are commonly referred to as brown tumors. These pseudotumors represent areas of fibrous scarring in which osteoclasts collect, blood decomposes, and cysts form. The most common sites of involvement are the mandible, clavicle, ribs, pelvis, and femur. Also, subchondral and subperiosteal bone resorption is invariably present. Kidney involvement results in nephrocalcinosis, impairment of renal function, and uremia.
thirst. The skeletal abnormalities most commonly seen are generalized osteopenia and foci of bone destruction, which are commonly referred to as brown tumors. These pseudotumors represent areas of fibrous scarring in which osteoclasts collect, blood decomposes, and cysts form. The most common sites of involvement are the mandible, clavicle, ribs, pelvis, and femur. Also, subchondral and subperiosteal bone resorption is invariably present. Kidney involvement results in nephrocalcinosis, impairment of renal function, and uremia.
Radiographic Evaluation
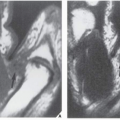
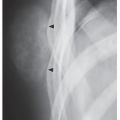
The major target sites in the skeletal system for hyperparathyroidism are the shoulder, the hand, the vertebrae, and the skull (Fig. 28.1). Conventional radiography is usually sufficient to demonstrate its characteristic features: generalized osteopenia; subperiosteal, subchondral, and cortical bone resorption; brown tumors; and soft-tissue and cartilage calcifications. Subperiosteal resorption is particularly well demonstrated on radiographs of the hands, where it usually affects the radial aspects of the middle phalanges of the middle and index fingers (Fig. 28.2; see also Figs. 26.7 and 26.9), although other bones can also be affected (Fig. 28.3). Commonly, subchondral bone resorption is present resulting in depression of overlying articular cartilage (Fig. 28.4). Also characteristic of this condition is resorption of the acromial ends of the clavicle (Fig. 28.5). Intracortical resorption is manifested by longitudinal striations, a finding known as tunneling, which can be most clearly appreciated on magnification studies (see Fig. 26.9B). Another characteristic feature is loss of the lamina dura around the tooth socket, which normally is seen as a thin sharp white line surrounding the peridental membrane that attaches the tooth to bone (Fig. 28.6). In the skull, there is a characteristic mottling of the vault, which yields a “salt-and-pepper” appearance (Fig. 28.7). Localized destructive changes in bones affected by hyperparathyroidism take the form of cystlike lesions of various sizes, commonly referred to as brown tumors. The jaw, pelvis, and femora are the usual sites for these lesions, but they may be found in any part of the skeleton (Fig. 28.8).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree