Hyperthermia
The medical manipulation of body temperature for treatment of disease has a long and fascinating history. Ancient references to the specific use of hyperthermia (or induced elevation of temperature above normal either locally, in part of the body, or of the whole individual) are found within the medical history of cultures from around the world. Modern interest in the cellular effects of heat energy by radiation oncologists was initially piqued by studies in the 1970s and 1980s demonstrating the highly quantifiable, time-dependent cytotoxic effects of “heat-shock” range temperatures (usually 41° to 45° C) on cells, similar to the highly reproducible and predictable effects of radiation. Moreover, hyperthermia in this temperature range was revealed to have clear additive, as well as synergistic, radiosensitizing properties. Although an early phase III trial in the United States in the 1980s testing the benefit of adding hyperthermia to radiation in patients with superficial tumors failed to show benefit, subsequent analysis of this trial revealed significant flaws in design, including the fact that many patients’ tumors were suboptimally heated at several of the participating centers. Indeed, since that time, multiple randomized trials examining the use of hyperthermia in combination with radiation and/or chemotherapy for a range of solid tumors, including deep tumors, have now been completed, demonstrating clear and significant clinical benefits. As a result, today, in some countries such as the Netherlands and Germany, hyperthermia is a fully approved and a reimbursable part of cancer care. The improved ability to control and measure thermal dose, in combination with a more complete recognition of the underlying biologic contribution of hyperthermia in radiochemosensitization, is currently driving a vigorous research effort, which will certainly further optimize the use of hyperthermia as an adjuvant for various oncologic and nononcologic disease targets.
For example, it is now appreciated that heating tumors in situ (and at temperatures as low as 39° to 42° C) can activate vascular, metabolic, and immunologic parameters of the tumor microenvironment, which may play an additional role in radiochemosensitization beyond hyperthermia-induced cell killing of tumor cells. Moreover, new functions for the focused delivery of heat in oncology are being identified, including as a highly specific means by which to concentrate cytotoxic drugs at the site of tumor using thermosensitive liposomes and other nanoparticles.
New image-guided approaches are now rapidly expanding the medical applications of thermal medicine. This includes the use of radiofrequency (RF) or ultrasound ablation technologies capable of focusing heat within tumors in situ to achieve very high (50° to 60° C) tumor temperatures over a very short interval, effectively instantly killing most tumor tissues without harming surrounding normal tissues. New nanoparticle technology including ferromagnetic fluids, nanotubes, and gold-coated particles are leading to completely new strategies for local heating of cells and tumors. In addition, this chapter also summarizes several recently completed clinical applications of hyperthermia with radiation and/or chemotherapy.
New image-guided approaches are now rapidly expanding the medical applications of thermal medicine. This includes the use of radiofrequency (RF) or ultrasound ablation technologies capable of focusing heat within tumors in situ to achieve very high (50° to 60° C) tumor temperatures over a very short interval, effectively instantly killing most tumor tissues without harming surrounding normal tissues. New nanoparticle technology including ferromagnetic fluids, nanotubes, and gold-coated particles are leading to completely new strategies for local heating of cells and tumors. In addition, this chapter also summarizes several recently completed clinical applications of hyperthermia with radiation and/or chemotherapy.
▪ CONTRIBUTION OF INDIRECT AND DIRECT EFFECTS OF HYPERTHERMIA TO RADIATION SENSITIZATION
Early Emphasis on the Direct Cytotoxic Effects of “Heat Shock” Temperatures
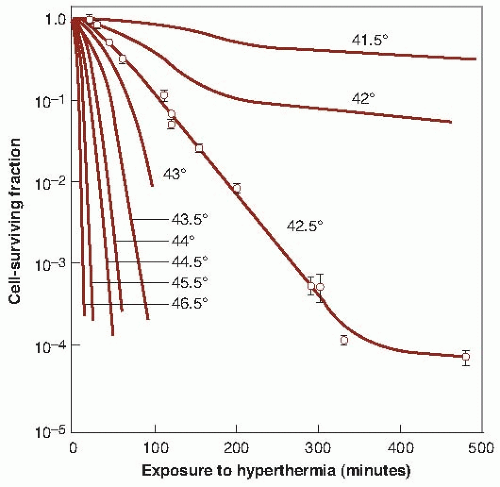
The research, which led directly to initial clinical trials during the 1980s and 1990s that aimed to locally heat patients’ tumors to temperatures to approximately 43° C, focused on the observation that tumor cell damage from focused heat energy that could add to, or even synergize with, the antitumor effects of radiation without the systemic toxicity typical of chemical radiation sensitizers. When cells are heated in vitro to a sufficient temperature and for a long enough duration, they die in a predictable, exponential manner, and the rate of killing increases with temperature. Figure 28.1 shows a series of survival curves for cells exposed for various periods to a range of temperatures from 41.5° to 46.5° C. The cell survival curves for heat are similar in shape to those obtained for x-rays (i.e., an initial shoulder followed by an exponential region), except that the time of exposure to the elevated temperature replaces the absorbed dose of x-rays. For lower temperatures, the picture is complicated, because the survival curves flatten out after a protracted exposure to hyperthermia, possibly indicating the development of “thermotolerance” or an acquired resistance or tolerance to the elevated temperature. The similarity in the shape of the cell survival curves for heat energy and x-rays is misleading. It is important, therefore, not to draw conclusions for heat based on the interpretation of radiation dose-response curves, because the amount of energy involved in cell inactivation is a thousand times greater for heat than for x-rays. This reflects the different mechanisms involved in cell killing by heat and x-rays. Families of survival curves similar to those in Figure 28.1 have been obtained for many different cell types, and it is clear that cells differ somewhat in their sensitivity
to heat-induced cellular damage in this temperature range. However, as with ionizing radiation, there is no consistent difference between normal and malignant cells heated in vitro.
to heat-induced cellular damage in this temperature range. However, as with ionizing radiation, there is no consistent difference between normal and malignant cells heated in vitro.
Survival data for Chinese hamster ovary cells exposed to various levels of hyperthermia (taken from Fig. 28.1) are replotted in Figure 28.2, with 1/D0 on the ordinate and 1/T on the abscissa. T is the absolute temperature; D0 is the reciprocal of the slope of the exponential region of the survival curve (i.e., the time at a given temperature that is necessary to reduce the fraction of surviving cells to 37% of their former value). This type of presentation is known as an Arrhenius plot; its slope gives the activation energy of the chemical process involved in the cell killing. The obvious change in slope, which is typical of Arrhenius plots, occurs at a temperature referred to as the “breakpoint” and is about 43° C for human cells. Above this temperature, an increase of 1° C doubles the rate of cell killing, and below the breakpoint, the rate of cell killing by heat drops by a factor of 2 to 4 for each drop of 1° C. The difference in activation energy above and below this temperature may reflect different mechanisms of cell killing (i.e., different targets for cytotoxicity above and below 43° C) or may reflect the development of thermotolerance within cells. Importantly, the slopes of Arrhenius plots derived from many in vitro and in vivo studies are nearly identical, and this consistency has provided long-standing usefulness of the Arrhenius analysis as a basis for assessing thermal dose in clinical hyperthermia applications up until the present time.
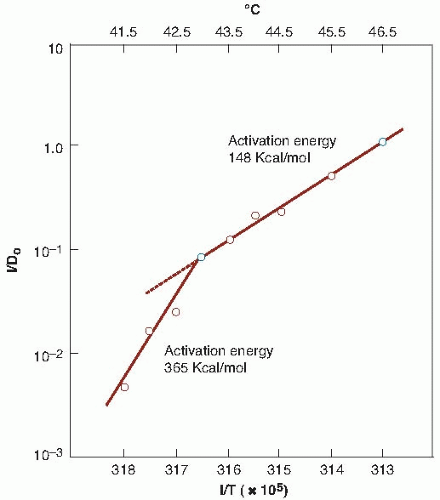 FIGURE 28.2 An Arrhenius plot for heat inactivation of mammalian cells in culture. The reciprocals of the D0 values obtained for Figure 28.1 are plotted versus the reciprocal of the absolute temperature. (Adapted from Dewey WC, Hopwood LE, Sapareto LA, et al. Cellular responses to combinations of hyperthermia and radiation. Radiology. 1997;123:463-474, with permission.) |
The similarity of the activation energy for protein denaturation to the activation energy for heat cytotoxicity, calculated from the Arrhenius analysis, led to the hypothesis that the target for heat cell killing in this temperature range resides in cellular proteins. Specifically, the heat of inactivation for cell killing and thermal damage is similar to the energy needed for protein denaturation (130-170 kcal/mol). Additional evidence for proteins as being the primary target for thermal cell killing is the importance of heat shock proteins (HSPs) in protecting thermotolerant cells from thermal damage. One of the primary functions of HSPs is to stabilize and refold other proteins that have been denatured or damaged, an event that signals their enhanced production. Structural chromosomal proteins, nuclear matrix, mitochondria and cytoskeleton, repair enzymes, transport proteins, and other membrane components are
all included among targets that have been shown to be denatured in a time-dependent manner by temperatures between 42° and 45° C. Enzymes in the respiratory chain are more heat sensitive than enzymes in the glycolytic pathway.
all included among targets that have been shown to be denatured in a time-dependent manner by temperatures between 42° and 45° C. Enzymes in the respiratory chain are more heat sensitive than enzymes in the glycolytic pathway.
Protein damage represents an important mechanistic difference between the manner in which heat and ionizing radiation lead to cellular damage, and this suggested that the two therapies could result in at least an additive ability to kill tumor cells if used together. There are other differences between irradiation and heating, suggesting that they could have additive effects. After irradiation, cells die only in attempting the next or a subsequent mitosis (except in very unusual circumstances), whereas heat-induced damage is expressed early, causing cells to die by various necrotic or apoptotic mechanisms, depending on the temperature. In addition, heat affects differentiating as well as dividing cells. Heat-induced chromosomal aberrations can occur if cells are in S phase during the time of heating. Although there is a familiar delay in x-ray response related to the time it takes for stem cells to progress through the process of differentiation and become functional, this delay is absent in the case of heat-induced cell death because all cells are affected, differentiated, or dividing; the thermal damage to the tissue is expressed immediately if the temperature is high enough. The DNA repair process itself is heat sensitive and this may be one of the mechanisms that lead to heat-induced radiosensitization and chemosensitization. Finally, although hypoxia renders cells resistant to radiation, it does not appear to affect sensitivity to heat.
Thermal Enhancement Ratio
The extent of the interaction of heat and radiation can be expressed in terms of the thermal enhancement ratio (TER), defined as the ratio of doses of x-rays required to produce a given level of biologic damage with and without the application of heat. The TER has been measured for various normal tissues, including skin, cartilage, and intestinal epithelium. The data form a consistent pattern of increasing TER with increasing temperature, up to a value of about 2 for a 1-hour heat treatment (HT) at 43° C. The TER is more difficult to measure in transplanted tumors in laboratory animals, because the direct cytotoxic effect of the heat tends to dominate. Heat often can control experimental tumors with acceptable damage to normal tissues, because cell killing by heat is strongly enhanced by nutritional deprivation and increased acidity, conditions that are typical of the poorly vascularized parts of solid tumors. Thus, a moderate HT, which can be tolerated by well-vascularized normal tissues, destroys a large proportion of the cells of many solid tumors in experimental animals. In those cases in which thermal radiosensitization has been studied, typical TER values are 1.4 at 41° C, 2.7 at 42.5° C, and 4.3 at 43° C, with heat applied for 1 hour. TERs have been developed for canine and human tumors however in studies by Gillette et al. and Overgaard et al. These estimates came from retrospective analyses of dose-effect relationships with and without hyperthermia. In canine oral squamous cell carcinomas, it was estimated to be approximately 1.15, when hyperthermia was administered twice a week during a course of fractionated radiotherapy. Importantly, the use of hyperthermia increased the slope of the TCD50 curve, such that the doses required to achieve a high level of local tumor control were maintained well below that which caused normal tissue damage (defined here as bone necrosis). TERs have also been estimated to be in the range of 1.5 for several superficial human tumor types.
Heat and the Therapeutic Gain Factor
The therapeutic gain factor can be defined as the ratio of the TER in the tumor to the TER in normal tissues. There is no advantage to using heat plus lower doses of x-rays if there is no therapeutic gain compared with the use of higher doses of x-rays alone. The question of a therapeutic gain factor is complicated in the case of heat, because the tumor and normal tissues are not necessarily at the same temperature. If the statement is made that heat preferentially damages tumor cells compared with normal tissue, it is implied that they are both at the same temperature. In a practical situation, however, this is not always the case. For example, if a poorly vascularized tumor is treated with microwaves, it may reach a higher temperature than the surrounding normal tissue, because less heat is carried away by the flow of blood. In addition, the overlying skin can be cooled actively by a draft of air or even a coldwater pack. In these circumstances, the normal tissues may be at a significantly lower temperature than the tumor, which therefore exaggerates the differential response in a favorable direction.
Factors that Modify Direct Cellular Damage from Hyperthermia
Because the earliest rationale for using heat as a radiation sensitizer involved maximizing direct heat-induced protein denaturation and cellular damage, there has been considerable attention by researchers given to factors that could modify tumor cell thermal sensitivity. For example, modification of cell membrane lipid content or use of membrane active agents, such as alcohols, was found to sensitize cells to heat killing, and the sensitization is probably related to destabilization of the membrane as it relates to lipid-protein interactions. Cells in an acid pH environment appear to be more sensitive to killing by heat. The pH dependence of cytotoxicity at elevated temperatures, however, is affected by pH history. Cells can adapt to pH changes and avoid the heat sensitivity shown for low pH.
Cells deficient in nutrients are quite heat sensitive. This can be demonstrated with cells in culture in which sensitivity to heat increases progressively as cells have their energy supply compromised, either by depriving them of glucose or by the use of a drug that uncouples oxidative phosphorylation. These conclusions about pH and nutrients, obtained under controlled conditions with cells in culture, led to the speculation that cells in tumors that are nutritionally deprived and at acid pH because of their location remote from a blood capillary may be particularly sensitive to heat. Because of their environment, it is likely that these cells are out of cycle and possibly hypoxic also. In this context, heat and x-rays would be predicted to be complementary in their action, because the cells that are most resistant to x-rays (out of cycle and hypoxic because of their remoteness from a capillary) show enhanced sensitivity to heat. A further enhancing factor that contributes to the complementary interaction of hyperthermia and radiation is that regions of a tumor in which the vasculature is poorly developed and potentially hypoxic tend to be at an elevated temperature because the cooling effect of blood flow is reduced.
Increased Recognition of the Importance of Indirect Effects of Hyperthermia: Heat and the Tumor Microenvironment
Some studies in mice had shown that local hyperthermia (using temperatures of 43° to 44° C) could naturally damage tumor vascular endothelium as well as tumor cells. This was predicted to lead to an increased accumulation of heat (because the damaged blood vessels would carry less heat away) while the pH, pO2, and nutrient status of the cells would decline, leading to enhanced tumor cell sensitivity to heat-induced cell killing. As a result of these studies, it was suggested that care was needed in combining heat and radiation, since tumor perfusion would be compromised by heating. For these reasons, several protocols have used hyperthermia in clinical applications after the first dose of radiation.
However, temperatures that can be achieved experimentally in mouse tumors are significantly higher than those which typically occur in human tumors under clinical conditions using locally applied, external heat energy; whether tumor vasculature in human tumors is damaged by the more modest temperatures reached under clinical conditions is still open to question. Further, it is important to also note that in animal studies in which more moderate temperatures are used (41° and 41.5° C), hyperthermia has been shown to promote tumor reoxygenation, with the degree of reoxygenation correlating with the level of the radiosensitivity of the tumor. Importantly, these data have been confirmed in a clinical study of patients with soft tissue sarcomas and breast cancer. Brizel and colleagues showed that one HT led to reoxygenation of human soft tissue sarcomas within 24 to 48 hours, whereas there was no measurable reoxygenation during a prior week of standard radiation therapy (RT). Jones and colleagues reported that mild hyperthermia (41° and 41.5° C at 90% of the measured points for 1 hour) significantly increased the pO2 in hypoxic, but not normoxic, human breast cancers. Such increases in tumor oxygenation could significantly improve tumor response to radiotherapy and is likely to be the primary important effect of local/regional or whole body forms of clinical hyperthermia. If this assumption is correct, then there should be much more attention devoted to optimal scheduling of hyperthermia in future trials to maximize oxygen-dependent radiosensitization. Indeed, instead of aiming to kill cells, mild hyperthermia may have the ability to rapidly stimulate global changes in the tumor microenvironment that collectively could enhance RT; this includes effects on pH, oxygen concentration, metabolism, protein/gene expression, and vascular perfusion.
It is well documented in human physiology that in normal tissues, a modest increase in temperature prompts highly sensitive thermoregulatory homeostatic mechanisms that increase heat dissipation in the vasculature. However, the ability to optimally thermoregulate is itself a highly temperature-dependent phenomenon. Optimal thermoregulation in terms of vascular effects occurs at mild to moderate hyperthermia and begins to fail above 41° and 42° C, resulting in heatstroke symptoms. While at temperatures of 43° C or higher, direct vascular and cellular damage can occur, and blood flow can be impaired. In cooler regions, the largest contributing factor to radiation sensitization by heating appears may be its ability to stimulate vascular activity, which, in turn, enhances delivery of additional oxygenated blood into tumors, sensitizing the tumor to radiation.
Despite an incomplete understanding of the actual basis of radiation sensitization by hyperthermia, an overestimation of the role of thermotolerance and suboptimal sequencing of hyperthermia and radiation fractions, multiple trials have been completed, revealing a critical role for hyperthermia in enhancing RT. A very active, preclinical, and clinical research effort is under way at the present time to develop the optimal dose and scheduling protocols and to design new clinical trials based on a more complete understanding of the physiologic effects of hyperthermia.
Thermotolerance
Exploration of the changing view of the role of “thermotolerance” in hyperthermia serves as a useful example demonstrating how our understanding of the radiosensitizing effects of hyperthermia have evolved over the past 2 to 3 decades. Thermotolerance, or induced thermal resistance, is usually described as the development of a transient and nonhereditary resistance to subsequent heating by an initial HT. Specifically, in vitro clonogenic assays of thermal cytotoxicity reveal that although one dose of heat kills a substantial fraction of cells, subsequent daily treatments are comparatively ineffective. Thermotolerance can begin to occur a few hours after the first treatment and may take as much as a week to decay. This would suggest that thermotolerance would be a significant problem clinically when heat is combined with fractionated radiotherapy, and most clinical trials were designed to minimize thermotolerance by keeping at least several days between hyperthermia fractions. However, it is now clear that in actual tumors in situ, an increase in blood flow and consequent increase in oxygenation by heating may significantly enhance the response of tumors to radiotherapy to a greater degree than any inhibition of thermal killing related to the occurrence of thermotolerance. Further, heat-induced radiosensitization is not subject to thermotolerance. Thus, although fears regarding thermotolerance had a historical importance regarding scheduling of hyperthermia for patients (schedules that may have turned out to be suboptimal in terms of achieving maximal benefits of hyperthermia), it is now clear that under normal heating conditions used in the clinic, thermotolerance may not be induced to any significant degree. Newer trials that are being designed to use more frequent applications of heating are likely to result in improved clinical benefit.
Another related area of research that has undergone considerable evolution in terms of understanding radiation sensitization by hyperthermia involves HSPs. It has been known for a long time that if cells are exposed to heat, proteins of a defined molecular weights or families are observed to be strongly induced above normal levels of expression. It was shown long ago that the appearance of these HSPs coincides with the development of thermotolerance, and their disappearance coincides with the decay of thermotolerance. Later molecular studies that specifically altered expression of certain HSPs demonstrated that they clearly exert a significant protective effect against potentially lethal heat exposures. Indeed, it has been long understood that HSP families are ubiquitous in evolution and are found in all living organisms. Similarly, a thermotolerance phenomenon also appears to be a conserved response in evolution resulting from their increased expression following exposure to certain stress conditions. However, although thermotolerance was one of the earliest functions attributed to HSPs, it is recognized today that these proteins play essential roles in numerous macromolecular processes under normal physiologic conditions as well as under stress conditions. In fact, there is now considerable research exploring induction of HSPs by hyperthermia treatments because of their likely role in promoting antitumor immune responses.
▪ IMMUNOLOGIC EFFECTS OF HYPERTHERMIA
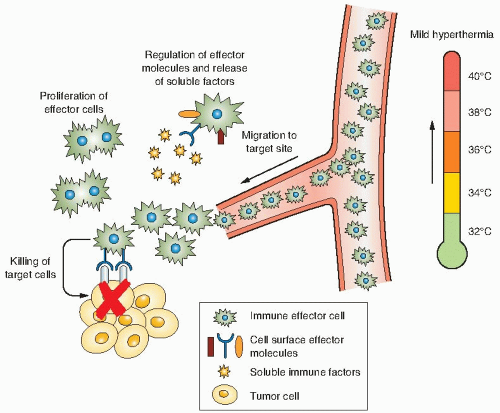
One of the most recent directions in the field of thermal medicine is the exploration of whether thermal stimulation of the antitumor immune response contributes to improved local control or long-term survival of patients receiving treatments of hyperthermia. Historically, some of the earliest attempts to use immunotherapy in cancer are linked to the medical origins of hyperthermic oncology, because it was reported that cancer patients who experienced the highest fevers following injection with infectious agents (i.e., Coley toxins) in an attempt to treat tumors may have also experienced the longest survival. Although there are major and important differences between hyperthermia and fever, both involve an upward shift in body temperature, a phenomenon that has been strongly correlated with improved survival following infection in multiple species. One possibility that is being considered is that clinical hyperthermia may trigger some of the same thermally sensitive targets in the immune system that have evolved over millions of years to respond to fever. An exciting possibility is that strategically applied HTs may serve as an adjuvant, helping to overcome at least some of the tumor escape mechanisms used by tumors to overcome immune recognition and destruction.
Although much more work is needed, current evidence that supports this possibility includes (1) enhanced immunogenicity and HSP expression seen after tumor cells are heated, (2) thermally enhanced immune effector cell activation and function, and (3) thermally enhanced vascular perfusion and delivery or trafficking of immune effector cells to tumors (Fig. 28.3). Current research goals in this rapidly growing field include definition of the role of thermal stress-induced HSPs in mediating enhanced T lymphocyte responses, and establishment of heating protocols in which thermally regulated immune effector mechanisms could be maximally
exploited. Although most hyperthermia applications have aimed to achieve temperatures of 42° to 45° C, or higher, normal fever-range body temperatures rarely exceed 40° C and are most often near 38° to 39° C. In fact, several studies demonstrate that cells of the immune system are more sensitive than other cell types to elevated temperatures. However, it should be kept in mind that even during thermal ablation, there are thermal gradients established at the margins of the tumor and in adjacent normal tissue where the temperature increase may be quite modest. And further, it would also be expected that there are extended regions of normal tissue that receive heated blood from the region of heated tumors; these distal draining tissues and blood cells may at a mildly elevated temperature for considerable lengths of time, long enough to provoke several relevant immunologic effects.
exploited. Although most hyperthermia applications have aimed to achieve temperatures of 42° to 45° C, or higher, normal fever-range body temperatures rarely exceed 40° C and are most often near 38° to 39° C. In fact, several studies demonstrate that cells of the immune system are more sensitive than other cell types to elevated temperatures. However, it should be kept in mind that even during thermal ablation, there are thermal gradients established at the margins of the tumor and in adjacent normal tissue where the temperature increase may be quite modest. And further, it would also be expected that there are extended regions of normal tissue that receive heated blood from the region of heated tumors; these distal draining tissues and blood cells may at a mildly elevated temperature for considerable lengths of time, long enough to provoke several relevant immunologic effects.
▪ MEASURING THERMAL DOSE IN PATIENTS
For every form of hyperthermia to be accurately delivered and evaluated in clinical trials, it is essential to achieve optimal quality control of heat delivery and temperature monitoring in patients. As was discussed previously, the temperature-induced cytotoxicity of tumor cells is dependent on both temperature and time, so that some type of time-integrated temperature analysis is considered the best approach to determining thermal dose. However, achieving this information in patients is not straightforward. Patients’ tumors differ in fundamental ways, including the ability to be heated, a parameter that may depend not only on physiologic factors such as blood flow, but also on body size, tumor positioning, and the power deposition of the heating device. Moreover, temperatures within a given tumors are not uniform, and hotter and cooler regions are seen (probably because of uneven vascular drainage), which limits the goal of reaching a uniform target temperature-time combination for therapy.
A major advance toward development of a tool by which to compare thermal dose among different patients was achieved when Sapareto and Dewey proposed to use the information obtained from the Arrhenius plot (which shows that there is a predictable relationship between the rate of cell killing and temperature) to help solve the problem of how to normalize the time-temperature data, which was seen to vary significantly from patient to patient. Sapareto and Dewey proposed the concept of “cumulative equivalent minutes” (CEM) at 43° C. More specifically, the measure of thermal dose stated as CEM 43° C T90 refers to the number of CEM at 43° C exceeded by 90% of the monitored points within the tumor. For this analysis, 43° C was chosen because it represents the breakpoint temperature for most human cells as judged from the Arrhenius plot. How does this idea work in practice? Because it is generally agreed that the effects of a 1° C rise of temperature is equivalent to a reduction of time by a factor of 2, consequently, above this transition temperature,
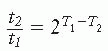
in which t1 and t2 are the heating times at temperatures T1 and T2, respectively, to produce equal biologic effect. For temperatures lower than the transition temperature, an increase in temperature by 1° C requires that time be decreased by a factor of 4 to 6:
The CEM 43° C T90, or the thermal dose, may be calculated from one of the other of these expressions or a combination of both: That is, the heat dose associated with a changing temperature may be calculated as the sum of equivalent heating times at 43° C for each temperature. Thus,
where CEM 43° C refers to the cumulative equivalent minutes at 43° C (the temperature suggested for normalization), t is the time of treatment, T is the average temperature during desired interval of heating, and R is a constant. When above the breakpoint, R is 0.5. When below the breakpoint, R is 0.25. For a complex time-temperature history, the heating profile is broken into short intervals of time “t” length (typically 1 to 2 minutes), where the temperature remains relatively constant. CEM 43° C is calculated for each interval and summed to give a final CEM 43° C for the entire HT.
CEM 43° C, also known as the “thermal isoeffect dose formulation, has now been used to
retrospectively evaluate heating efficacy in several clinical trials. Moreover, two recent phase III clinical trials by Jones et al. and Thrall et al. used this formulation to prospectively evaluate patients’ tumors to determine whether they were “heatable” prior to randomization. Further, this concept was used to define a thermal dose, which could identify patients who received a higher thermal dose from those which received a lower dose. These trials have been extremely important for showing that not all tumors are heatable to the same degree and the ability to actually control of thermal dose delivery can affect the probability for response and duration of local tumor control. This concept is of critical importance for future clinical success of hyperthermia and is clearly an area deserving of additional research. Moreover, using the concept of CEM 43° C, these trials have clearly established that the actual temperatures achieved in patients’ tumors is less than the target temperature of 43° C for a significant portion of time, over a significant portion of tumor. Nevertheless, significant clinical benefit is obtained. These data are creating a major current paradigm shift with regard to what may actually be happening in the tumor microenvironment that promotes radiation sensitivity, because the temperatures achieved would appear to be lower than that required for thermal cytotoxicity.
retrospectively evaluate heating efficacy in several clinical trials. Moreover, two recent phase III clinical trials by Jones et al. and Thrall et al. used this formulation to prospectively evaluate patients’ tumors to determine whether they were “heatable” prior to randomization. Further, this concept was used to define a thermal dose, which could identify patients who received a higher thermal dose from those which received a lower dose. These trials have been extremely important for showing that not all tumors are heatable to the same degree and the ability to actually control of thermal dose delivery can affect the probability for response and duration of local tumor control. This concept is of critical importance for future clinical success of hyperthermia and is clearly an area deserving of additional research. Moreover, using the concept of CEM 43° C, these trials have clearly established that the actual temperatures achieved in patients’ tumors is less than the target temperature of 43° C for a significant portion of time, over a significant portion of tumor. Nevertheless, significant clinical benefit is obtained. These data are creating a major current paradigm shift with regard to what may actually be happening in the tumor microenvironment that promotes radiation sensitivity, because the temperatures achieved would appear to be lower than that required for thermal cytotoxicity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree