Molecular Imaging
▪ RADIOLOGIC IMAGING
The challenge of noninvasively mapping biologic tissue has occupied radiologists and engineers for over a century. Shortly after Roentgen’s discovery of the x-ray in 1895, it was applied toward medical imaging by directing x-rays through his wife’s hand and onto a piece of film, which revealed the bony structure of her hand. In the time since that landmark discovery, medical imaging has evolved to encompass multiple modalities and mechanisms of signal generation and reconstruction. X-ray radiography matured over the first half of the 20th century, while the first medical ultrasonic measurement was made in 1954, followed by the invention of the Anger camera in 1964 and the advancement of nuclear medicine, the introduction of the clinical x-ray computed tomography (CT) scanner in 1972, the acquisition of the first magnetic resonance image (MRI) in 1973, and the introduction of the first clinical positron emission tomography (PET) device in 1975. These methods have developed to offer high imaging performance in terms of scanning time, spatial resolution, and image quality, and now are commonplace in medical settings.
Imaging techniques were first exploited by the radiation oncologist for the identification and targeting of tumors to be irradiated. With the use of radiographic imaging to localize a neoplastic mass within a patient, radiotherapy can be more accurately delivered to the intended target while sparing surrounding tissues. Furthermore, as radiation therapy physics progressed toward the calculation of delivered doses using patientspecific information, three-dimensional x-ray CT was used as a model of patient structure. This modality is especially well suited to radiotherapy planning as the image intensities reflect x-ray absorption and can therefore be used to accurately predict dose deposition. For these reasons, x-ray radiography and CT are now essential tools in the radiation oncology clinic. As MRI methods have advanced, this imaging modality and its superior soft tissue contrast relative to x-ray CT have also become incorporated into many radiation oncology departments. However, a shortcoming of these modalities is that their source of contrast is gross tissue anatomy or structure. X-ray CT differentiates tissues based on their density and x-ray absorption, whereas MRI detects differences in proton density and magnetic relaxation. These properties are not directly related to the physiologic or functional aspects of the tissue being imaged. This limitation of conventional imaging prompted the development of molecular imaging, generally defined as the noninvasive detection, localization, and quantification of specific molecular entities or physiologic processes in a living organism.
Over the last 20 years, the field of molecular imaging has undergone tremendous growth, with significant advances in the interrelated areas of imaging technology, imaging target identification, molecular probe development, and image analysis.
As such, these imaging methods are now being adopted into the practice of radiation oncology to better stage and plan tumors for treatment and to monitor their response to therapy. Despite encouraging preliminary results of studies employing molecular imaging in the diagnosis, staging, and treatment of cancer, many challenges remain to be overcome to fully exploit the potential of this technology in this setting. The imaging methods with the most utility for oncology must be identified, and quantitative and/or volumetric metrics that can be extracted from these images must be established and incorporated into the staging, treatment planning, and posttreatment monitoring of radiation oncology patients. Many molecular imaging modalities have been engineered, including nuclear medicine techniques, optical imaging, and functional MRI methods including fMRI and magnetic resonance spectroscopy; however, this chapter will focus on two imaging techniques with widespread utility in radiology and radiation oncology: x-ray CT and PET. Although CT is commonly used as an anatomic imaging modality, developments with x-ray contrast agents and dynamic acquisition protocols have allowed the collection of functional information with this imaging method. Alternately, PET imaging of radioactive imaging agents has reached a point where this technique is now routinely being used to complement conventional imaging. The physical aspects of each of these modalities will be summarized, after which their role in clinical imaging will be described, including discussion of their use in clinical tumor staging, treatment planning, and follow-up.
As such, these imaging methods are now being adopted into the practice of radiation oncology to better stage and plan tumors for treatment and to monitor their response to therapy. Despite encouraging preliminary results of studies employing molecular imaging in the diagnosis, staging, and treatment of cancer, many challenges remain to be overcome to fully exploit the potential of this technology in this setting. The imaging methods with the most utility for oncology must be identified, and quantitative and/or volumetric metrics that can be extracted from these images must be established and incorporated into the staging, treatment planning, and posttreatment monitoring of radiation oncology patients. Many molecular imaging modalities have been engineered, including nuclear medicine techniques, optical imaging, and functional MRI methods including fMRI and magnetic resonance spectroscopy; however, this chapter will focus on two imaging techniques with widespread utility in radiology and radiation oncology: x-ray CT and PET. Although CT is commonly used as an anatomic imaging modality, developments with x-ray contrast agents and dynamic acquisition protocols have allowed the collection of functional information with this imaging method. Alternately, PET imaging of radioactive imaging agents has reached a point where this technique is now routinely being used to complement conventional imaging. The physical aspects of each of these modalities will be summarized, after which their role in clinical imaging will be described, including discussion of their use in clinical tumor staging, treatment planning, and follow-up.
▪ X-RAY COMPUTED TOMOGRAPHY
Imaging of biologic tissues with electromagnetic radiation is among the oldest medical imaging methods and has a long history in its application to oncology. Although two-dimensional x-ray radiographs were first applied to the visualization of tissues of interest, the continued advancement of x-ray imaging technology has resulted in the widespread adoption of both two-dimensional x-ray fluoroscopy as well as three-dimensional x-ray CT in the modern oncology clinic.
Basic Physics
X-ray Radiography and Computed Tomography
Consider a beam of x-rays passing through the chest of a patient, striking a film on the far side. Ignoring scatter in this treatment, one can consider the x-ray beam to be made up of several smaller, parallel beams, each passing through specific tissue volumes on their way to the film. Because each tissue volume has its own x-ray absorption properties depending on the atomic composition of the tissue, the image formed on the film will vary in brightness depending on how the patient attenuated the x-ray beam at different spatial locations. This concept of measuring differential x-ray absorption is the basis of all x-ray imaging methods. If the measurement geometry and the intensity of the source are known, the average mass attenuation coefficient (µ/ρ) along the path traversed by each x-ray path may be calculated. In biologic subjects, bony tissues are typically strongly absorbing and have high µ/ρ for x-rays at the energy of typical imaging systems, whereas low-density tissues, such as lungs, are poorly absorbing and have low µ/ρ.
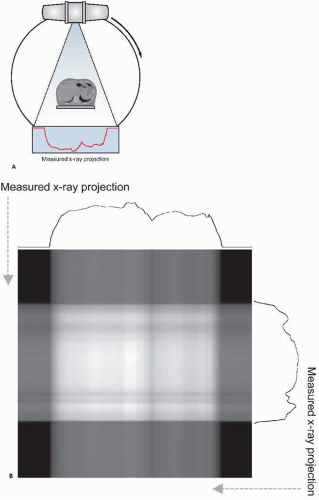
Two-dimensional x-ray radiography is commonly used for various medical applications, including the diagnosis of bone fractures, pneumonia and lung diseases, and congestive heart failure. This technique has been extended using fast digital detectors to allow dynamic imaging of a subject, known as x-ray fluoroscopy. This method facilitates image-based monitoring of dynamic physiologic processes such as in angiography, the assessment of interventional procedures such as device implantation, and precise patient positioning such as that needed for radiotherapy setup. However, two-dimensional x-ray imaging collapses all information along the third dimensions, providing a projection through a subject instead of resolving objects in three dimensions. Several situations may not be amenable to this technique, for example, in the detection of a lung lesion that is masked by highly absorbing ribs in the same x-ray projection. To overcome this limitation, three-dimensional x-ray imaging methods employing tomography have been developed. The mathematics of x-ray tomography were elucidated as early as the 1930s. Tomography involves the acquisition of multiple two-dimensional projection views of a subject, with each view acquired through a different projection angle as demonstrated in Figure 15.1A. These views are then combined using a tomographic reconstruction algorithm to mathematically resolve the three-dimensional structure of the subject. X-ray CT has matured through
several data collection geometries and reconstruction methods since its introduction in 1972; however, the fundamental tomographic concept remains the same, as does the source of contrast (x-ray attenuation) in the images.
several data collection geometries and reconstruction methods since its introduction in 1972; however, the fundamental tomographic concept remains the same, as does the source of contrast (x-ray attenuation) in the images.
Imaging Hardware
X-ray imaging and CT employ an x-ray source that produces a beam that is directed through a subject, and an x-ray detector that measures several x-rays that traverse the subject. The geometry of the CT scanner has evolved through several forms as this imaging modality has been developed. Hounsfield’s original scanner, known as “first generation” CT, included only one or two spatial detector channels, which therefore measured x-ray penetration through the subject at only a few discrete locations. This detector setup, paired with a tightly collimated x-ray beam that only irradiated these detectors, was translated across the patient to measure x-ray fluence along a single projection angle at multiple locations. After recording this projection, this apparatus was rotated and the process repeated to record a new projection. CT scanners have evolved greatly since this original instrument, with wide-angle x-ray sources and detectors minimizing the time required to collect a full set of tomographic projections. In most modern CT scanners, the source and detector are mounted on a rotating gantry as depicted in Figure 15.1A.
The most common x-ray source used in imaging systems is the x-ray tube. This device is a type of vacuum tube, consisting of a cathode that generates electrons and an anode toward which the electrons are accelerated through a voltage applied across the cathode/anode pair. The anode absorbs the energy of the accelerated electrons and emits a portion of it in the form of x-rays. The x-rays produced are known as Bremsstrahlung or braking radiation, because the incident electrons are slowed and the energy lost is emitted in the form of an x-ray. The emitted x-rays range in energy from near zero to a maximum corresponding to the voltage applied to the tube. Generally, the mean photon energy produced by an x-ray tube is approximately onethird of the voltage applied to the tube. X-rays produced by the anode are then emitted through a window in the vacuum tube and collimated to a desired beam profile. The beam is commonly also “filtered” by the insertion of thin sheets of metal such as copper or aluminum in the beam path. This has the effect of preferentially removing low-energy photons from the beam and increasing the average energy of the beam. Beam profiles for CT scanning are generally either fans (wide angular dispersion, narrow profile) designed to function with a correspondingly narrow one-dimensional detector array, or cones (rectangular profile) for irradiation of a rectangular planar two-dimensional detector array.
The simplest x-ray detector is radiographic film, which darkens upon absorption of x-rays. However, the need for a reusable, digital detector capable of rapid and repeat imaging has encouraged the development of electronic x-ray detectors. Although detectors such as scintillators that convert x-rays into visible photons and subsequently to electrical signals are common, many modern x-ray imaging systems now employ solid-state semiconductor detectors because they are capable of direct digital detection of x-rays without the need for conversion of the incoming photons into an intermediate form, such as visible photons. Research is ongoing to engineer new detector materials capable of measuring very small numbers of photons to reduce the dose required for x-ray imaging. In modern CT scanners, multiple detector rows are now commonly used to collect multiple CT slices in a single acquisition, resulting in scanners generally referred to as multidetector CT (MDCT). These advances have resulted in scan times for x-ray CT as short as 0.5 seconds per frame. Helical scanning protocols, in which the subject is translated longitudinally through the CT ring as the gantry rotates and projection data are recorded, has further accelerated the acquisition of volumetric CT data. In addition to advances in fan beam architectures for CT, cone beam scanners have emerged following the development of high-performance flat panel imaging arrays and effective cone beam volumetric reconstruction methods, and are now being integrated in many linear accelerator designs to facilitate the collection of CT data during radiotherapy.
Image Reconstruction and Analysis
The most common reconstruction algorithm applied to x-ray CT data is the filtered backprojection technique, a form of the inverse radon transform. This method uniformally distributes each measured projection back along the line
from which it was acquired, as demonstrated in Figure 15.1B. This results in an image of the original object when sufficient projections are used. Because this technique introduces a blurring into the reconstructed image, the projections are sharpened through application of a ramp filter in the frequency domain prior to backprojection. Because of the filter that is used, filtered backprojection is sensitive to high- frequency noise, which commonly necessitates the use of smoothing to reduce noise at the cost of spatial resolution. To overcome the shortcomings of this method, several iterative techniques have been proposed that improve reconstruction performance at the cost of increased computational complexity. Iterative routines function by modeling the data acquisition process and applying this model to an initial guess at the reconstructed image distribution, producing a set of simulated measurements. These simulations are then compared to the actual measurements and the reconstructed image is correspondingly adjusted. This process is then repeated until there is acceptable agreement between the measured and simulated data. Although computationally expensive, these methods allow sophisticated modeling of the data acquisition process, permitting consideration of scatter, noise, and other factors.
from which it was acquired, as demonstrated in Figure 15.1B. This results in an image of the original object when sufficient projections are used. Because this technique introduces a blurring into the reconstructed image, the projections are sharpened through application of a ramp filter in the frequency domain prior to backprojection. Because of the filter that is used, filtered backprojection is sensitive to high- frequency noise, which commonly necessitates the use of smoothing to reduce noise at the cost of spatial resolution. To overcome the shortcomings of this method, several iterative techniques have been proposed that improve reconstruction performance at the cost of increased computational complexity. Iterative routines function by modeling the data acquisition process and applying this model to an initial guess at the reconstructed image distribution, producing a set of simulated measurements. These simulations are then compared to the actual measurements and the reconstructed image is correspondingly adjusted. This process is then repeated until there is acceptable agreement between the measured and simulated data. Although computationally expensive, these methods allow sophisticated modeling of the data acquisition process, permitting consideration of scatter, noise, and other factors.
Pixel intensities in reconstructed CT images are typically converted into the Hounsfield unit (HU) scale, a standardized intensity range constructed to permit comparison of x-ray opacity between images and scanners. In this scale, all measured x-ray absorption values µ are related to that of water µwater by the relation
This is a linear function; therefore, HUs are proportional to the measured x-ray absorption µ. Examining the given expression, it can be seen that the HU of water is 0, whereas the HU of air, with µ ≈ 0, is – 1,000. Water and waterbased soft tissues demonstrate HU of 0 to 100, whereas fatty tissues occupy the HU range – 100 to 0. Strongly absorbing bones exhibit HU of 500 or greater. Modern CT scanners are capable of detecting differences in HU on the order of 5 to 10 HU.
X-ray Contrast Agents
Agents that alter x-ray absorption after delivery to a subject have been developed nearly as long as x-ray imaging itself to improve on the minimal x-ray contrast between soft tissues. Because of the linear relationship between concentration and x-ray attenuation, and between x-ray attenuation and HU, the effectiveness of an x-ray contrast material is dictated by the x-ray absorption of its constituent materials and the concentration at which it can be delivered. Strongly absorbing elements including barium (Z = 56), gadolinium (Z = 64), gold (Z = 79), bismuth (Z = 83), and tungsten (Z = 74) have been studied as potential x-ray contrast materials; however, iodine (Z = 53) has emerged as the predominant component of clinical x-ray contrast agents because of its biocompatibility and its ease of incorporation into labeling chemistries. Sodium iodide was administered as an oral contrast agent for x-ray imaging in 1918, and subsequently various iodine-containing compounds were engineered to include more iodine per mole, improve pharmacokinetics, and reduce toxicity. Although ionic compounds prevailed through the first half of the 20th century, the synthesis of nonionic multi-iodinated agents such as iopamidol and iohexol in the 1970s greatly reduced the toxicity of iodinated contrast agents, and these have become clinical standard. Gases including krypton (Z = 36) and xenon (Z = 54) have also been used as inhaled x-ray contrast agents. However, the limiting factor with all x-ray contrast agents is the large concentrations required to produce a measurable change in imaging signal. For iodine, the concentration required to produce a CT change of 100 HU is approximately 10 mM.
Nanomaterials
The development of nanotechnology over the last 10 years has encouraged engineering of new x-ray contrast materials by overcoming the limitations of previous contrast agents. Nanoparticles facilitate the use of otherwise toxic or biologically incompatible materials in contrast agent formulations, while also permitting the packing of a large number of contrast-producing atoms in a single composite structure. In addition, the large surface area and functionalized status of many nanoparticle constructs allows the conjugation of other components to the nanoparticle to alter molecular targeting, biodistribution, and/or pharmacokinetics.
Iodine-based or iodine-containing nanoparticles have been a topic of intense interest, with
multiple synthetic strategies having emerged including highly iodinated fullerenes, polymeric macromolecules containing trioiodinated moieties, crystalline iodinated nanoparticles dispersed with surfactant, and polyethylene glycol (PEG)-based shells enclosing iodinated materials at high concentrations.
multiple synthetic strategies having emerged including highly iodinated fullerenes, polymeric macromolecules containing trioiodinated moieties, crystalline iodinated nanoparticles dispersed with surfactant, and polyethylene glycol (PEG)-based shells enclosing iodinated materials at high concentrations.
More recently, significant work has been performed to evaluate the use of gold nanoparticles for CT contrast. Hainfield and colleagues showed that small (˜100 µm) blood vessels can be visualized within minutes of intravenous delivery of gold nanoparticles. As with iodine nanoparticles, PEG has also been incorporated into gold nanoparticles to alter their pharmacokinetics. Although concentration limits have hampered the use of contrast CT for molecular imaging, the use of gold nanoparticles in this context has been examined in vitro through the coupling of UM-A9 antibodies specific to head and neck squamous cell carcinoma to gold nanorods. Although measurements of cells with and without the target antigen demonstrated a fivefold difference in HU, it remains unclear whether this level of contrast will be sufficient in vivo. Targeting of gold nanoparticles to folic acid receptor has also been investigated as a second molecular-specific CT imaging agent.
Nanoparticles synthesized from bismuth sulphide have also recently been evaluated for in vivo CT vascular imaging. This agent has been seen to be tolerable to mice and rabbits at a dose containing 0.2 M bismuth, giving CT numbers in excess of 500 HU in vivo. Toxicity for bismuth nanoparticles was seen to be significantly less than equivalent concentrations of bismuth salts. Other heavy metals including gadolinium, dysprosium, erbium, europium, and lutetium have been incorporated into fullerene structures to produce water-soluble x-ray contrast materials. These molecules represent additional pathways toward novel x-ray contrast materials that may yet evolve into valuable tools for CT imaging.
Radiotherapy Dose Enhancement
A potentially useful secondary property of x-ray contrast materials is their ability to enhance the cell kill produced by high-energy radiation. This effect is mediated by the electrons produced on photoelectric absorption of high-energy photons by high-Z materials and is produced both by endogenous materials such as bone as well as exogenous contrast agents. Although pair production for megavoltage photons commonly used in radiotherapy may also enhance radiation dose effect, even at this energy range, photoelectrons are the primary cause of this enhancement. This enhancement has been studied by several groups through simulations and animal experiments; however, it has a limited history of clinical application with only preliminary studies of kilovoltage radiotherapy of human brain tumors after intravenous injection of an iodinated contrast agent having been conducted. Recent developments with nanoparticle technology may yet exploit this phenomenon, however.
Clinical Applications of X-ray Computed Tomography
Radiotherapy Planning
One of the principle tasks of radiotherapy planning is the delineation of patient anatomy, including the desired radiation target as well as organs at risk that should be spared from radiation dose. These identified volumes are then used to compute an optimal radiation treatment strategy, employing the degrees of freedom available to the specific radiotherapy treatment device. X-ray CT has become the primary imaging modality used for this process, both because of its high spatial and temporal resolution and lack of image distortion artifacts, as well as the fact that CT image intensities denote x-ray absorption and can be used to predict dose deposition by a treatment beam within the patient. However, the low CT contrast between soft tissues complicates the task of the radiation oncologist and has raised interest in use of other imaging modalities such as PET to improve radiotherapy planning. This is discussed further in the subsequent paragraphs.
Vascular and Perfusion Imaging
Because of the physiologic and clinical significance of the vasculature within tumors, efforts to image this aspect of the cancer microenvironment have been widespread. With CT, one mechanism to achieve this goal is the intravenous delivery of a contrast agent followed by repeat imaging as the agent passes through the vasculature. Through analysis of the collected images, various parameters including blood flow, blood volume, and vascular permeability may be derived. This method is generally called dynamic contrast-enhanced (DCE) imaging. In its
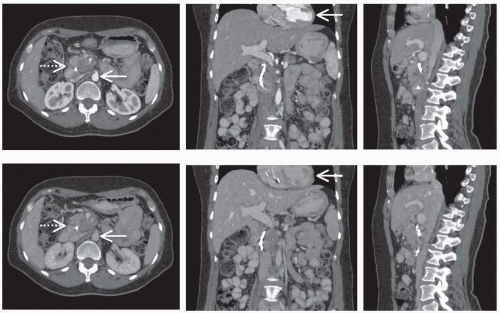
simplest form, this method can be applied following an intravenous bolus contrast injection to acquire images timed to when the contrast material is passing through a specific portion of the vasculature. For example, commonly contrast-enhanced CT includes the acquisition of an “arterial phase” image as the agent is passing through the arterial system, followed by a later “venous phase” image. These images may then be considered either independently or together to assess vascular structure and function, as shown in Figure 15.2. More sophisticated DCECT approaches however involve the acquisition of images with greater temporal resolution over a longer period before and after contrast injection. The signal curve obtained for a given pixel over time may be analyzed in several ways to estimate specific vascular parameters. Empirical assessment of the CT signal versus time curve has been evaluated, examining measurements such as the maximum uptake of contrast agent, the time to reach maximum uptake, or the maximum slope of the initial signal rise, and many of which have been used to identify and characterize malignant lesions. Alternately, several quantitative models that describe the pharmacokinetics of contrast agents have been developed. These models can be fit to experimentally measured signal curves to estimate the parameters of the model, which may include vascular volume, perfusion, vascular permeability, and vascular surface area depending on the specific model and acquisition sequence. This imaging protocol and analysis is demonstrated in Figure 15.3.
simplest form, this method can be applied following an intravenous bolus contrast injection to acquire images timed to when the contrast material is passing through a specific portion of the vasculature. For example, commonly contrast-enhanced CT includes the acquisition of an “arterial phase” image as the agent is passing through the arterial system, followed by a later “venous phase” image. These images may then be considered either independently or together to assess vascular structure and function, as shown in Figure 15.2. More sophisticated DCECT approaches however involve the acquisition of images with greater temporal resolution over a longer period before and after contrast injection. The signal curve obtained for a given pixel over time may be analyzed in several ways to estimate specific vascular parameters. Empirical assessment of the CT signal versus time curve has been evaluated, examining measurements such as the maximum uptake of contrast agent, the time to reach maximum uptake, or the maximum slope of the initial signal rise, and many of which have been used to identify and characterize malignant lesions. Alternately, several quantitative models that describe the pharmacokinetics of contrast agents have been developed. These models can be fit to experimentally measured signal curves to estimate the parameters of the model, which may include vascular volume, perfusion, vascular permeability, and vascular surface area depending on the specific model and acquisition sequence. This imaging protocol and analysis is demonstrated in Figure 15.3.
Tumor Diagnosis, Staging, and Response Assessment
X-ray CT has become a routine clinical procedure in the management of oncology patients. Lung tumors are particularly suited toward diagnosis with x-ray imaging methods because of the large contrast between low-density, air-filled
lung tissue and solid lung tumor masses. Fast CT scanning technology as well as methods for managing breathing motion, including breath-hold CT and four-dimensional (time-resolved) CT, have further improved the utility of this imaging modality for interrogating thoracic lesions and especially for delineating spatial targets for radiotherapy. The high spatial resolution of x-ray CT has encouraged the use of CT-based measures of tumor volume as a prognostic marker. However, the usefulness of this measure is limited for small nodules, which has encouraged the use of contrast-enhanced CT to evaluate malignancy.
lung tissue and solid lung tumor masses. Fast CT scanning technology as well as methods for managing breathing motion, including breath-hold CT and four-dimensional (time-resolved) CT, have further improved the utility of this imaging modality for interrogating thoracic lesions and especially for delineating spatial targets for radiotherapy. The high spatial resolution of x-ray CT has encouraged the use of CT-based measures of tumor volume as a prognostic marker. However, the usefulness of this measure is limited for small nodules, which has encouraged the use of contrast-enhanced CT to evaluate malignancy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree