CHAPTER 8
Hypoplasia of the Right Ventricle
Definition
Pulmonary Atresia with Intact Ventricular Septum
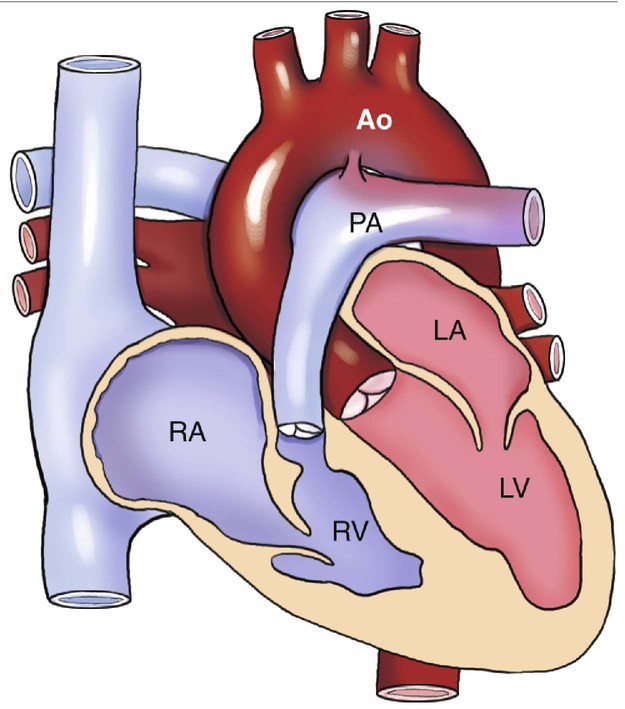
Pulmonary atresia in this malformation is always valvular1 and, in conjunction with the intact ventricular septum, severely reduces blood flow to the right ventricular chamber, thus impeding its ability to develop properly (Fig. 8–1).2,3 Pulmonary atresia with intact ventricular septum may also result in a normal or enlarged right ventricle, but both are less common.4,5
Recent data reported a hypoplastic right ventricle in 80% of cases. A normal ventricle is present in 6.5%, and in 13% the ventricle is enlarged.5,6
Right ventricular hypoplasia is classified on the basis of the tripartite approach originally described by Goor and Lillehei.7,8 The normal right ventricle is divided into a sinus (inlet) portion, incorporating the tricuspid valve apparatus; an apical trabecular portion lying beyond the insertion of the papillary muscles of the tricuspid valve toward the apex; and a conus (infundibulum) portion leading to the pulmonary valve.8
Classifications included the following:
The pulmonary valve tissue in this abnormality is described as a membrane with fused commissures depicted by raphae and with three formed cusps.8 Rarely is the valve bicuspid or without leaflets.9,10
The tricuspid valve is, by definition, patent in all cases of pulmonary atresia with intact ventricular septum, but it is rarely normal.1 The tricuspid valve orifice is usually proportional to the size of the right ventricular cavity.9,11,12 The valve leaflets are usually dysplastic and result in varying degrees of insufficiency or stenosis.
The right atrium may be dilated, depending on the degree of tricuspid regurgitation, as may the foramen ovale. In some cases of right atrium dilatation, bulging of the interatrial septum can obstruct the mitral valve.6 Severe restriction of the foramen ovale has been reported in 10% of cases.13
The aortic valve is usually normal; however, rare cases of aortic stenosis have been described.14 The left ventricle is often dilated and hypertrophied secondary to increased blood volume resulting from hemodynamic changes. The left atrium, mitral valve, and pulmonary veins are usually unaffected.
Tricuspid Atresia
Tricuspid atresia also results in a hypoplastic right ventricle but should be considered a separate anomaly from pulmonary atresia with intact ventricular septum. Tricuspid atresia is defined as complete agenesis of the tricuspid valve, resulting in the absence of direct communication between the right atrium and the right ventricle.15,16
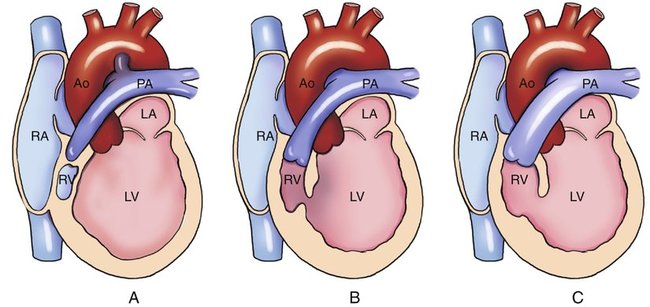
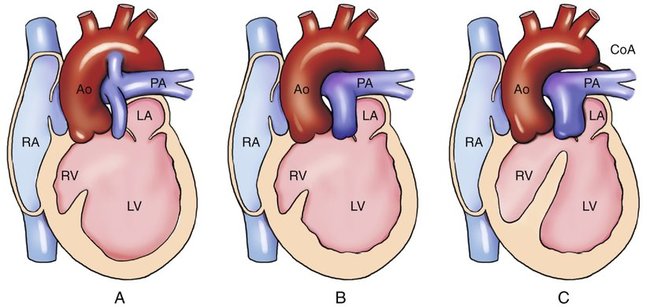
Tricuspid atresia is divided into three types on the basis of the relationship of the great arteries (Table 8–1). In type I, the relationship of the great vessels is normal; it accounts for approximately 70% of cases of tricuspid atresia at autopsy. In 28% of cases, there is dextro (d)-transposition (complete transposition) of the great arteries (type II), and levo (l)-transposition (corrected transposition) of the great arteries (type III) occurs in 3% of cases.15 These three types are subclassified on the basis of the presence or absence and size of the associated ventricular septal defect (VSD) and on the presence or absence of pulmonary atresia or stenosis (Figs. 8–2 and 8–3).
TABLE 8–1
| Type | Relationship | Frequency (%) |
| I | Normal great vessels | 69 |
| IA | No VSD, pulmonary atresia | 9 |
| IB | Restrictive VSD, pulmonary atresia | 51 |
| IC | Nonrestrictive VSD, no pulmonary stenosis | 9 |
| II | Dextrotransposition of great arteries | 28 |
| IIA | VSD, pulmonary atresia | 2 |
| IIB | VSD, pulmonary stenosis | 8 |
| IIC | VSD, no pulmonary stenosis | 18 |
| III | Levotransposition of great arteries | 3 |
Adapted from Rosenthal A, Dick M: Tricuspid atresia. In: Adams FH, Emmanouilides GC, Riemenschneider TA (eds): Moss’ Heart Disease in Infants, Children, and Adolescents, 4th ed. Baltimore, Williams & Wilkins, 1989, p 903.
Weinberg described five morphological variants of the tricuspid valve associated with tricuspid atresia17:
1. Muscular atresia: The most common type, occurring in 76% to 84% of cases. No tricuspid valvular tissue is found, and the floor of the right atrium is muscular.
2. Membranous atresia: The membranous portion of the atrioventricular septum, between the right atrium and the left ventricle, forms the floor of the right atrium. Four percent to 12% of patients have membranous atresia.
3. Valvular atresia: Six percent of cases involve a right atrial floor composed of a thin, imperforate membrane of valvular tissue.
4. Ebstein form: Valvular tissue in the floor of the right atrium is adherent to the right ventricular wall, forming an “atrialized” portion of the right ventricle. Seen in 4% to 6% of cases.
5. Common atrioventricular canal: The rarest form (2%), consisting of a leaflet of a common atrioventricular valve completely sealing the entrance to the right ventricle.
Aneurysmal dilatation of the foramen ovale due to right atrial dilatation occasionally occurs, resulting in obstruction to left atrial blood flow.18,19 A true atrial septal defect (ostium secundum) is present in about one third of cases. Less commonly, a primum atrial septal defect, or complete absence of the atrial septum, may occur.20 As with pulmonary atresia with intact ventricular septum, the mitral valve and left atrium are usually normal, and the left ventricle is enlarged and hypertrophied as a result of increased blood volume.
The degree of right ventricular hypertrophy associated with tricuspid atresia is dependent on the anatomical type of atresia and the size of the VSD.21 However, it is smaller than normal in all cases and is usually described as “slitlike.”
Embryology
Pulmonary atresia with intact ventricular septum is postulated to occur after cardiac septation.1 Kutsche and Van Mierop10 suggested that this defect might result from a prenatal inflammatory disease rather than being a true congenital malformation. Although little data exist to support this theory, the association of pulmonary artery obstruction with maternal rubella infection has been reported.22,23
Occurrence Rate
Pulmonary atresia with intact ventricular septum accounts for 1% to 5% of cases of congenital heart disease.5,11,24,25 It occurs in 0.1 to 0.4 in 10,000 live births.26 Pulmonary atresia with intact ventricular septum has been reported to occur slightly more frequently in males.8
Tricuspid atresia has been reported in 0.3% to 3.7% of patients with congenital heart disease.14 The prevalence at autopsy has been reported to be between 2% and 3%.27–29 Tricuspid atresia occurs in approximately 1 in 15,000 live births.27,28 There does not appear to be a sex predilection; however, cases associated with transposition of the great arteries occur more commonly in males.16,30,31
Sonographic Criteria
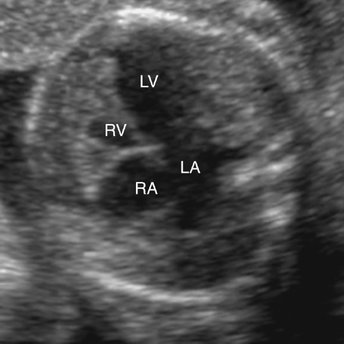
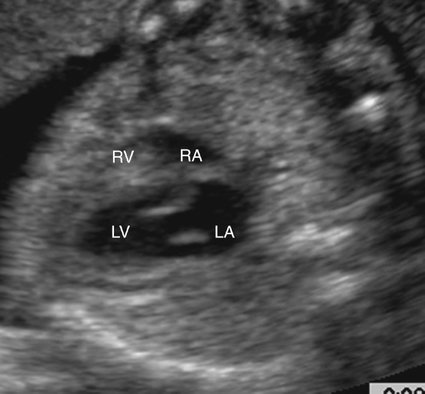
Hypoplasia of the right ventricle can be diagnosed on fetal echocardiography by identifying a small right ventricular chamber. This is best accomplished in an apical (Fig. 8–4) or subcostal four-chamber view (Fig. 8–5). Careful attention is necessary to identify the small right ventricle and avoid confusion with a univentricular heart.
The size and patency of the tricuspid valve should be addressed with use of color and pulsed Doppler imaging. In tricuspid atresia, no flow will be detected on either side of the valve (Figs. 8–6 and Fig. 8–7). The valve and valve orifice appear hyperechoic and thickened (Fig. 8–8). In pulmonary atresia with intact ventricular septum, the tricuspid valve may also appear small and hyperechoic, but some blood flow should be detected. If the tricuspid valve is stenotic, Doppler interrogation distal to the valve shows an increase in velocity and, often, turbulent flow. The tricuspid valve is often insufficient in cases of pulmonary atresia with intact ventricular septum. In this setting, regurgitant flow should be apparent by placing the pulsed Doppler cursor proximal to the valve in the right atrium (Fig. 8–9). Because pulsed Doppler tracings of regurgitant flow may be of a velocity high enough to cause aliasing, color Doppler imaging is often a more effective means of quantitating the amount of regurgitant flow present (Fig. 8–10).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree