CHAPTER 7
Hypoplastic Left Heart Syndrome
Definition
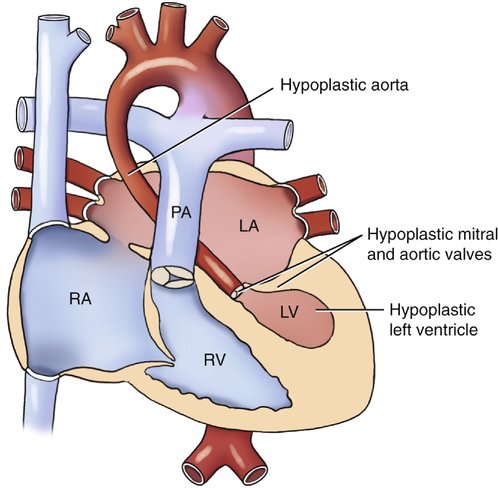
Hypoplastic left heart syndrome (HLHS) refers to a spectrum of cardiac abnormalities that includes underdevelopment of the left ventricle, mitral valve, aorta, and aortic valve.1–3
This syndrome is the most severe form of left-sided obstructive lesions, and it is among the most severe forms of congenital heart disease (CHD).4–8 It consists, in varying degrees, of a small left ventricle associated with aortic atresia, a hypoplastic ascending aorta, mitral valve atresia or hypoplasia, and a small left atrium (Fig. 7–1).
The terms “aortic atresia” and “HLHS” are often used in reference to the same lesion; however, these two anomalies are not synonymous because a small percentage of patients with aortic atresia have a normal left ventricle.9
Embryology
The early developmental changes that result in a hypoplastic left ventricle are not completely understood.10 Several hypotheses currently exist.4
Decreased ventricular size has been achieved in animal models by reducing the blood flow through the foramen ovale.11 The development of the cardiac chambers is dependent on the flow of blood through them. Therefore decreased perfusion of the left atrium and left ventricle may result in hypoplastic chambers, aortic valve atresia, mitral valve atresia, and hypoplasia of the aortic arch.4,12
Malalignment of the interatrial septum toward the left has also been proposed as a mechanism of interfering with left ventricular filling, resulting in poor development of that chamber.13
Another explanation for HLHS is that fusion of the endocardial cushions occurs but fails to produce a valve.12,13
It may also be due to an overgrowth of the ventral atrioventricular cushion, which deprives the ascending aorta of adequate blood flow.14 The resultant change in flow leads to the development of the structural cardiac anomalies associated with this syndrome.12,15
Part of the interventricular septum and the ventricular muscle develops from this atrioventricular canal; papillary muscles and chordae tendinae arise from the ventricular muscle.10
A third hypothesis is that the causative factor is a rudimentary left atrium and, consequently, diminished blood flow to the mitral portion of the atrioventricular canal. The left ventricle and aorta or aortic valve thus fail to develop properly because of the decreased blood flow through the lumen.10
The left ventricle will be small, the aortic valve may be stenotic, and coarctation of the aorta may result.12,16 Aortic stenosis or atresia may be valvular, subvalvular, or supravalvular. Subvalvular and valvular types often occur together.14,17
The ductus arteriosus is patent; however, the foramen ovale may close completely in utero with resultant severe hypoplasia of the left ventricle.18
The ventricular septum is generally intact but may be deviated into a subaortic position.
Conduction disturbances may be present in HLHS because of an interruption in the bundle of His.12,16,19–21
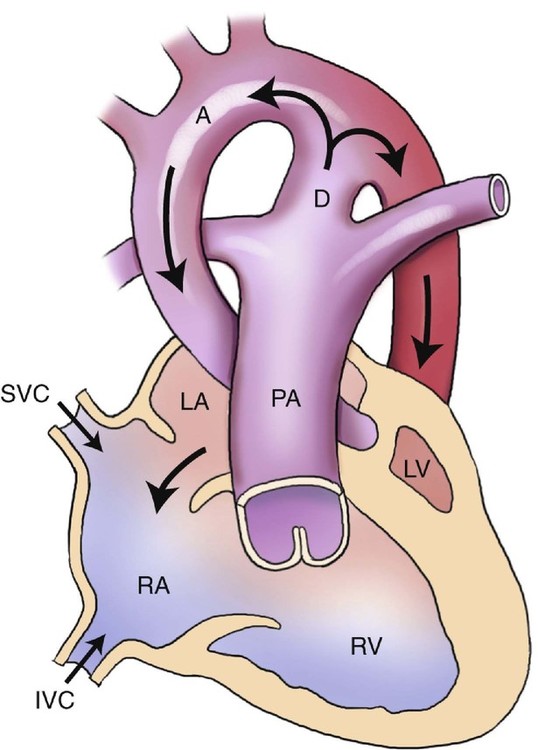
Hemodynamically, blood is unable to pass through the left ventricle and aortic valve and therefore is shunted in a retrograde manner from the left atrium, through the foramen ovale, and back into the right atrium (Fig. 7–2). This results in an increase in the size of the right atrium, right ventricle, and main pulmonary artery.
Because of the left heart hypoplasia, the right ventricle supplies both the pulmonary and systemic circulations. The pulmonary veins may be either normal or increased in size. The blood volume and pressure in the right heart are elevated.22 The left atrium, left ventricle, and aorta are all decreased in size. In severe cases of hypoplasia of the ascending aorta, the coronary arteries are perfused in a retrograde manner by the ductus arteriosus.6,23
HLHS results in cardiac function similar to that seen in univentricular hearts. The increased right ventricular pressure, together with inadequate flow out of the left atrium, results in elevated pulmonary venous pressure and pulmonary congestion.16
Occurrence Rate
HLHS is the most common cause of death from CHD in the early neonatal period, accounting for 7% to 9% of all structural heart defects and 25% of all cardiac-related deaths.3,24 HLHS comprises 13% of all prenatally diagnosed CHD. There appears to be a male dominance, especially in the presence of atresia of the aortic valve.20–22,25,26
Most cases of HLHS are likely multifactorial. However, multiple studies suggest a genetic basis as the etiology of many cases. Approximately 19% of affected patients with HLHS have a first-degree relative with CHD, reinforcing the case for a genetic link to HLHS.4,27,28 It is thought to have an autosomal recessive inheritance pattern.
The risk of occurrence has been reported to be 2% in siblings of an affected child.24,25,28–30
Sonographic Criteria
HLHS is easily recognized in utero. It should be borne in mind, however, that HLHS is a progressive lesion and may not manifest itself until the late second trimester. Studies show early screening may be advantageous but does not obviate the importance of second-trimester fetal echocardiography.3,31
First trimester nuchal translucency screening has increased early detection of HLHS. Studies suggest a stronger correlation between left-sided obstructive lesions and a thickened nuchal translucency. A measurement greater than 2.5 multiples of the median is associated with an increased risk for CHD, including HLHS and should be an considered an indication for fetal echocardiography.3,32
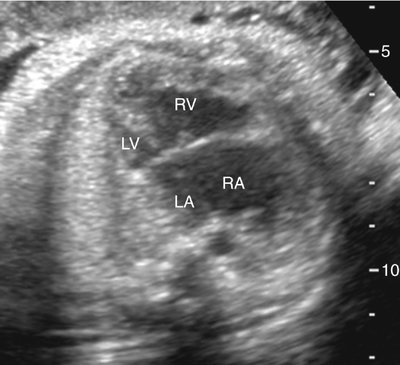
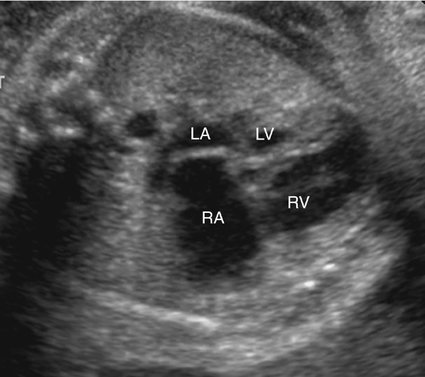
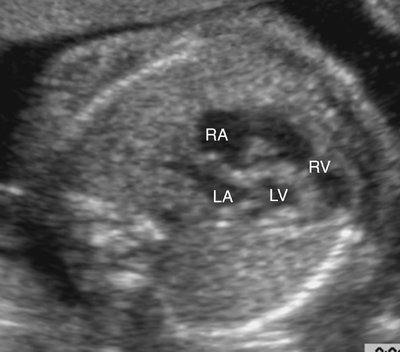
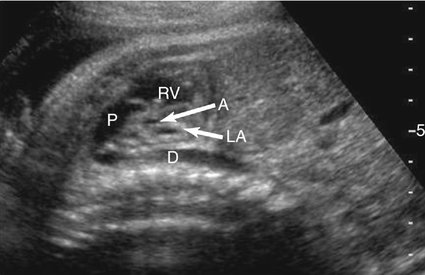
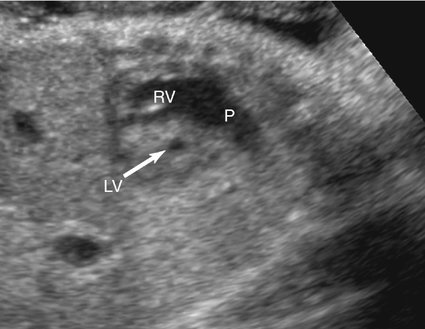
The majority of patients with HLHS maintain a normal cardiac axis. An apical (Fig. 7–3) or subcostal (Fig. 7–4) four-chamber view of the heart can be used to show the size discrepancy of the ventricles. It is important to identify the morphological features of the right ventricle, including the moderator band and the tricuspid valve, to confirm it is the left ventricle that is hypoplastic. In HLHS, the right ventricle constitutes the cardiac apex, giving rise to the term “apex-forming ventricle” (Fig. 7–5).21,22,33
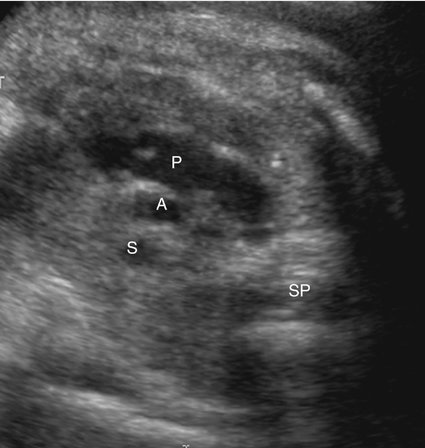
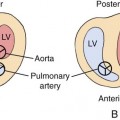
A short-axis view of the great vessels (Fig. 7–6), a long-axis view of the aorta (Fig. 7–7), or a three-vessel view (Fig. 7–8) can be used to assess the size of the ascending aorta. An atretic aorta can be difficult to visualize because of its small size. On the other hand, the pulmonary artery is often enlarged. Sonographically, an atretic aorta and aortic valve can appear hyperechoic in addition to being small.
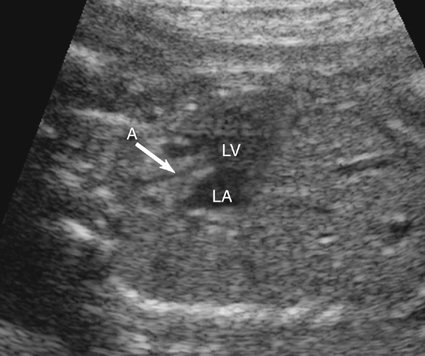
The presence and size of the left ventricle can be evaluated in a short-axis view of the ventricles (Fig. 7–9) and either four-chamber view.
Anomalous pulmonary venous connection is not uncommon with HLHS. The connection of the pulmonary veins to the left atrium should be determined in either four-chamber view. The pulmonary veins are often enlarged as a result of pulmonary congestion. Color or pulsed Doppler imaging is useful in confirming their normal connection the left atrium.29
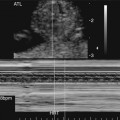
A restrictive foramen ovale is one of the worst prognostic signs associated with HLHS. Therefore a thorough assessment of the interatrial septum is mandatory. Color or pulsed Doppler imaging should be used to confirm the presence and direction of blood flow through the foramen ovale (Fig. 7–10). If the interatrial septum is seen bowing to the left, either anomalous pulmonary venous drainage or severe tricuspid regurgitation may be present.29
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree