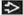Idiopathic Pulmonary Fibrosis
C. Nicholas Fetko, MD
Aqeel A. Chowdhry, MD
Tan-Lucien H. Mohammed, MD, FCCP
Key Facts
Terminology
Idiopathic interstitial pneumonias of unknown cause
Associated with histologic pattern of usual interstitial pneumonia on surgical biopsy
Imaging Findings
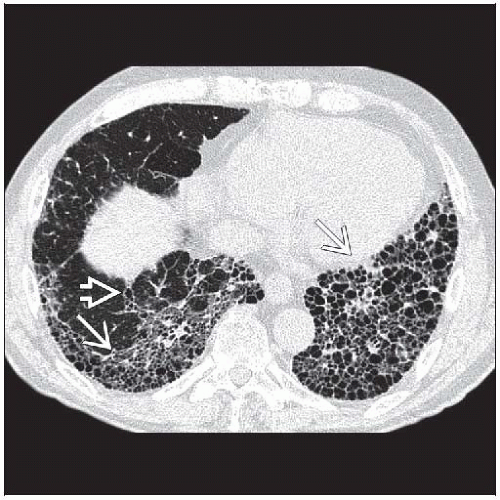
Subpleural and basal honeycombing and traction bronchiectasis
Reticular opacities > > ground-glass opacities
Consider alternative diagnosis to IPF if centrilobular nodules, cysts, extensive ground-glass opacities, consolidation, peribronchovascular distribution
Acute exacerbation
New ground-glass opacities or consolidation
Peripheral (60%), diffuse (25%), multifocal (15%)
Positive predictive value of confident diagnosis = 95-100%
Top Differential Diagnoses
Nonspecific Interstitial Pneumonitis
Asbestosis
Chronic Hypersensitivity Pneumonitis
Drug Reaction
Pathology
Usual interstitial pneumonia histologic pattern characterized by spatial and temporal heterogeneity
Clinical Issues
Median survival following diagnosis about 3.5 years
Transplantation only effective treatment
Diagnostic Checklist
Drug reaction in any patient with IPF pattern
TERMINOLOGY
Abbreviations and Synonyms
Idiopathic pulmonary fibrosis (IPF), cryptogenic fibrosing alveolitis (CFA), usual interstitial pneumonia (UIP)
Definitions
Most common form of idiopathic interstitial pneumonias
Associated with histologic pattern of usual interstitial pneumonia on surgical biopsy
IMAGING FINDINGS
General Features
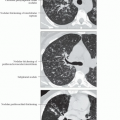
Best diagnostic clue: Subpleural and basal honeycombing and traction bronchiectasis
Patient position/location
Subpleural and basilar lung
Costophrenic angles should be most affected portion of lungs
Morphology
Reticular opacities, honeycombing, traction bronchiectasis
Relative lack of ground-glass opacities
CT Findings
Morphology
Predominant reticular pattern with honeycombing
Peripheral honeycomb cysts may vary in size, dependent on phase of respiratory cycle
Traction bronchiectasis or bronchiolectasis essential to diagnosis
Ground-glass opacities
Reticular opacities > > ground-glass opacities
Minor component of overall pattern
Emphysema: Coexistent centrilobular or paraseptal emphysema in about 30%
Pulmonary ossification may occur in long-standing fibrosis
Consider alternative diagnosis to IPF if centrilobular nodules, cysts, extensive ground-glass opacities, consolidation, peribronchovascular distribution
Distribution
Basal and peripheral predominance
Patchy, heterogeneous distribution abuts normal lung
May be asymmetric, but not unilateral
Mean extent of disease 25% ± 10%
Volume loss prominent with honeycombing
Pulmonary artery enlargement
Reflects pulmonary artery hypertension seen in 50% of those undergoing pre-transplant evaluation
Other findings include right ventricular wall hypertrophy, dilatation of right ventricle and atrium, contrast reflux into inferior vena cava, and hepatic veins
Adenopathy (50%)
Mediastinal lymph node enlargement < 2 cm short axis diameter
Most common location right paratracheal nodal station (American Thoracic Society [ATS] region 4) or subcarinal (ATS region 7)
Frequency related to extent of fibrosis and duration of disease
Complications
Acute exacerbation
Morphology: New ground-glass opacities or consolidation
Distribution: Peripheral (60%), diffuse (25%), multifocal (15%)
Generally spares honeycomb lung
Bronchogenic carcinoma
Seen in up to 10% of patients with long-standing IPF
Typical appearance: Focal consolidation in periphery of lower lobes in area of severe fibrosis
May be multiple
Barotrauma: Increased risk for pneumothorax, pneumomediastinum
Infection: Higher risk for mycobacterial species, Aspergillus, and other organisms
Suspect infection with progressive consolidation or cavitation
Accuracy
Should make confident diagnosis in 50% of cases
Positive predictive value of confident diagnosis = 95-100%
Interestingly, interobserver agreement of presence of honeycombing only about 70%
Radiographic Findings
Radiography: Not as specific as CT but usually abnormal
Imaging Recommendations
Best imaging tool: HRCT to detect and characterize interstitial lung disease
DIFFERENTIAL DIAGNOSIS
Nonspecific Interstitial Pneumonitis
Ground-glass opacities > > reticular pattern
Traction bronchiectasis out of proportion to extent of reticular opacities
Bronchovascular distribution more common
Honeycombing not as prominent
Traction bronchiectasis extends into more central airways with IPF
Asbestosis
Subpleural reticular pattern with honeycombing identical to IPF
Fibrosis in asbestosis may be coarser than in IPF
Subpleural curvilinear lines, mosaic perfusion, and parenchymal bands more common
Associated (calcified) pleural plaques
Bronchiolectasis more common in IPF
Chronic Hypersensitivity Pneumonitis
Inferior costophrenic angles not most severely involved lung (usually mid and upper lung)
Centrilobular nodules, lobular air-trapping more common
Rheumatoid Arthritis
Systemic Sclerosis
Basilar predominant pulmonary fibrosis
Dilated esophagus not seen with IPF
Subcutaneous calcifications distinguish from IPF
More frequently associated with NSIP pattern
Drug Reaction
Can cause similar radiographic abnormalities
Typical drugs: Nitrofurantoin (Macrodantin) and chemotherapy drugs
Sarcoidosis
Typically causes bronchocentric fibrosis in upper lung zones
Some have pattern indistinguishable from IPF
Can be associated with mediastinal and hilar adenopathy