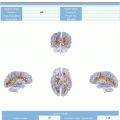
Fig. 4.1
Zoom of the signal intensity curves showing signal change during first pass of contrast bolus acquired with four different sampling rates (4, 5, 6, and 7 s)
Another key factor is the transformation of signal intensity changes into contrast concentration changes. In imaging modalities like CT or PET, there is a linear relationship between signal changes and contrast concentration changes. However, in other modalities like MR, this relation is not linear and needs to be carefully taken into account during image acquisition and image processing. On top of that, these signal changes cannot be directly obtained from the signal, and some basal calibrations are required for a proper quantification. As a result, from the nonlinearity between the MR signal and the contrast concentration, the effect of contrast over singal changes depends on the baseline T1 values of the tissues. For those tissues with short baseline T1 values, the MR signal change due to contrast uptake will be smaller than for tissues with longer baseline T1 values. Figure 4.2 shows a simulation of the effect of contrast on the MR signal in two different tissues, equally perfused but with different baseline T1 values (260 and 1500 ms). Figure 4.2b shows the normalized signal with a relative signal difference of 80 % between both tissues. As both tissues have the same perfusion value and contrast concentration, the difference is just derived from different baseline T1 values. To compensate the impact of baseline T1 differences on quantitative MR perfusion, some baseline measurements must be acquired during the imaging acquisition protocol; this way reproducibility between different scans and patients will be improved. These baseline T1 measurements are generally addressed using special image acquisition MR sequences [19, 20].


Fig. 4.2
Simulation of contrast uptake in two tissues with different baseline T1 values. Both tissues have equal perfusion and contrast arrival. (a) Signal intensity during the first pass of the contrast normalized by the signal before contrast arrival (S0). (b) Signal intensity change compared with baseline signal (S0), normalized by the baseline signal
During the contrast administration, a dynamic protocol must be acquired. This dynamic protocol must be defined to enhance the image contrast to provide a more precise analysis. In certain medical imaging modalities, such as CT, besides improving contrast sensitivity, special attention has to be paid to reduce the radiation exposure. Iterative reconstruction techniques have a big impact on both aspects [17, 21], allowing to reduce the total radiation dose with minor impact on image quality while maintaining excellent contrast sensitivity [17].
MR image contrast depends on many different acquisition parameters that need to be properly tuned and combined. For dynamic contrast-enhanced experiments, it is required to enhance the T1 properties of the contrast, avoiding at the same time potential signal saturations. A good review of the technical details and the process to assess perfusion parameters by MR can be found in [22]. For cardiac perfusion, different approaches have been proposed to avoid signal saturation due to high contrast concentration, especially in the assessment of VIF while maintaining adequate uptake in the cardiac muscle [18, 23, 24]. In other organs, such as the prostate, the standard approach is to use 3D acquisitions covering the whole organ. On those sequences, spoiled gradient echo sequences are generally applied; signal modeling is perfectly described in these sequences, which ensures a proper conversion from signal variation to contrast concentration changes. In the case of 3D acquisitions, in order to avoid T1 saturation of the signal caused by high contrast concentrations, relative high excitation pulses are usually applied with flip angles between 20 and 30°. In addition to the T1 effect, it is important to reduce the impact of T2* in VIF estimation. To avoid this T2* effects, it is recommended to acquire the images with minimum TE. In the QIBA protocol for MR perfusion biomarker, a TE lower than 1.5 ms is proposed as ideal and a range between 2 and 2.5 ms as acceptable. In the case of the repetition time (TR), the ideal values would be lower than 3 ms, while values between 5 and 7 ms are acceptable.
Conclusions
In this chapter we have introduced the rationale to define an imaging protocol to generate imaging biomarkers from medical images; this rationale has been illustrated using different practical examples.
Accuracy and reproducibility of any biomarker are the key properties that make it suitable to be used in clinical decisions. To fulfill these requirements, it is essential to choose the right medical image modality that must ensure high sensitivity to the physiological property that wants to be measured, minimizing at the same time potential artifacts in the final measurements. Once the image modality is selected, the full biomarker protocol needs to be defined; this protocol must cover from patient preparation to imaging protocol definition. Patient preparation needs to fulfill all safety aspects related to the imaging test and to collect all the physiological information required in the post-processing step. Finally, the image acquisition protocol needs to be adjusted to gather all the information that is required by the post-processing to provide accurate outcomes, avoiding any potential confounding factor.
References
1.
O’Neill RT. FDA’s critical path initiative: a perspective on contributions of biostatistics. Biom J. 2006;48(4):559–64.CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree