1
Revision sinus surgery
2
Distorted sinus anatomy of development, postoperative, or traumatic origin
3
Extensive sino-nasal polyposis
4
Pathology involving the frontal, posterior ethmoid, and sphenoid sinuses
5
Disease abutting the skull base, orbit, optic nerve, or carotid artery
6
CSF rhinorrhea or conditions where there is a skull base defect
7
Benign and malignant sino-nasal neoplasms
Practical Examples
Radiation Dose Exposure and Extensive Application of CAS-CT in Rhinosurgery: Example of Implementation of Radiation Dose Reduction in Clinical Practice
Elective imaging should be performed only after maximal topical and antibiotic therapies have been attempted in order to document residual disease which cannot be further treated without surgery. Although CAS based on MR is feasible [12], preoperative acquisition of CT-based datasets for navigation is still standard.
Today’s imaging resolution reaches pixel and voxel sizes in the millimeter (MR) and submillimeter (CT) range, which is rarely insufficient for CAS. In our experience of the last 5 years, failure to use CT datasets from referring radiological suites was most often due to inadequate image boundaries (not depicting the whole region of interest: ROI), followed by inadequate image slice thickness (exceeding 0.5 to 1 mm in the axial plane [13]), and finally, poor resolution accounting for 57, 29, and 14 % of rejected cases respectively [14].
Current CT technology provides the surgeon with detailed and often excessive picture quality. However, this detailed information has an associated cost of radiation exposure and radiation-induced health hazards (cataract formation, optic neuropathy, and radiation-induced malignancy). According to European Community and American guidelines, radiation dose reduction is a constant concern because of the inflationary growth of CT examinations [15], which leads to the principle of keeping dose exposure as low as reasonably achievable. In rhinology, dose-reduced CT scanning protocols for uncomplicated sinusitis do exist [16]. However, only recently was radiation dose reduction assessed for CT-based navigation in FESS for clinically applied surface matching [17] and preclinical experiments with rigid fixed fiducials [18] (Fig. 63.1).
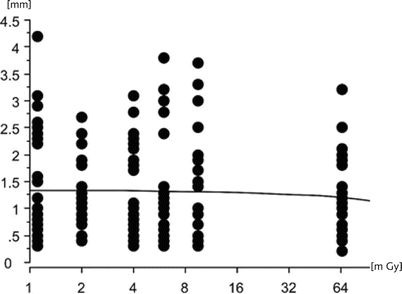

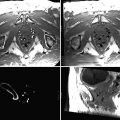
Fig. 63.1
Scatterplot of accuracy measurements in millimeters for screw-marked points depicted on the y-axis. CAS-CT dose used in milligrays depicted on the logarithmic x-axis. Each column represents the accuracy in millimeters for seven screw-marked points on four cadaveric heads for registration with CAS-CT at a given dose. The regression line shows no clinically significant slope for accuracy with exponential growth of the radiation dose in the dose interval tested (Nauer et al. with permission [18])
These studies showed that the differences in radiation absorption between tissue and air are large enough to be resistant to diminishing signal-to-noise ratio and blurring of the CT image. Thus, bone window-based CAS in FESS represents a particularly good opportunity for dose reduction if the need for high tissue contrast or contrast media application can be excluded, i.e., for the majority of surgical cases with uncomplicated chronic rhinosinusitis. Although exact surface matching is feasible, dose reduction leads to noisier pictures, which are subjectively perceived by each surgeon differently, thus radiation dose cannot be reduced at all costs. With an approximate effective dose of 0.25 mSv as compared to standard chest X-ray imaging (0.02–0.05 mSv), digital volume tomography (DVT) currently offers another option for reduction of radiation exposure compared to older CT scanners. As a low-dose technology, DVT is excellent in imaging of radiopaque implants and bony contours yet lacks adequate soft tissue contrast comparable with gradients of the Hounsfield scale of high dose CT. Modern DVT hardware is small and easy to use, which even facilitates intraoperative imaging in high-risk surgery to track soft tissue shift, imprecise resection, or complication [19].
Computer-Aided Minimally Invasive Biopsy of Cavernous Sinus Tumor Infiltrating the Pterygoid Fossa
In endoscopic sinus surgery, only limited view of anatomy is afforded, and the use of CAS systems aims at avoiding the injury of important anatomical structures around the orbit and the anterior skull base. Especially critical are the internal carotid artery, the optic nerve, and the floor of the anterior skull base [20]. While these systems lead to an increased intervention time [21] and the need for preoperative imaging, they improve orientation in narrow anatomical compartments allowing precise localization and controlled tissue sampling in situations with highly altered anatomical landmarks and scarring. In special cases, even soft tissue processes localized to high-risk areas such as the cavernous sinus can be sampled by its extension into the pterygoid fossa. Transnasal endoscopic biopsy by a maxillary sinustomy type II with limited trephination of the backwall of the maxillary sinus enabled controlled tissue sampling above the main branch of the internal maxillary artery at the roof of the pterygopalatine fossa. Tissue sampling relied wholly on imaging, as macroscopically the tumor could not be clearly depicted in this area. Results of the biopsy revealed invasive non-differentiated squamous cell carcinoma originating from a lung cancer with a metastasis to the cavernous sinus (Fig. 63.2).
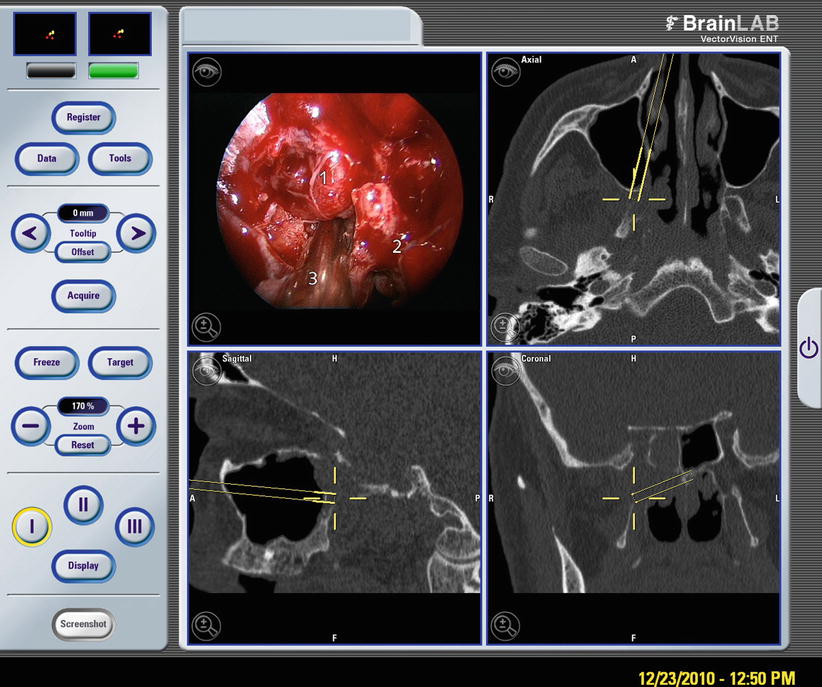

Fig. 63.2
Image-guided biopsy of squamous cell carcinoma extending from the cavernous sinus into the pterygoid fossa. Below a pulsating branch of the internal maxillary artery (1) next to tumor mass (2), navigated suction tip (3)
Intraoperative Control of Transsphenoidal Drainage of Clivus Cholesterol Granuloma Documenting Controlled Brain Stem Decompression
Image fusion of CT- and MR-based data in preoperative surgical planning takes advantage of both image modalities by combining optimal depiction of bony contours (CT) with soft tissue delineation (MR). Not only does the capacity of today’s computers enable more data to be processed in a reasonable amount of time for use preoperatively, but CT or MR imaging and image processing is also more commonly available directly in the OR. Even before completing an intervention, the results of surgery, e.g., the radicality of tumor resection and residual disease, soft tissue shifts, and adversary side effects like intracranial tension pneumatocephalus or bleeding, can be diagnosed without time delay. For example, fusion of pre- and post-interventional data concerning the drainage of a large cholesterol with MR is feasible, depicting directly the amount of brain stem decompression, ruling out decompression bleeding, brain herniation, or pneumatocephalon (Fig. 63.3). In addition to intraoperative MR suites, recently developed mobile digital volume tomography will become more readily available and may become an integrated part of future clinical routine for maxillofacial and rhinological surgery.

Fig. 63.3
Endoscopic transsphenoidal decompression of clivus cholesterol granuloma. Endoscopic view of the opened dorsal wall of the sphenoid with shimmering cholesterol flakes in the drained granuloma fluid (upper left). Fused preoperative MRI and MRI at the end of surgery. Segmented blue area (1) depicting preoperative extension of the cholesterol granuloma, segmented yellow area (2) documents the final result of adequate drainage and brain stem decompression with neither brain herniation nor decompression bleeding
Endonasal Operation with Augmented Reality Endoscopic Systems (ARES)
Augmented reality (AR) allows the user to see the real world, with virtual objects superimposed on it. Therefore, an AR system will enhance the endoscopic view and further enable surgeons to view hidden critical structures such as pathologies (e.g., tumors), risk regions or sensitive structures (e.g., arteries or nerves), or the results of a preoperative planning, such as pathways, trajectories, or distances [22]. A CT-based planner enables the surgeon to select important anatomical landmarks, as well as target risk structures. Such landmarks are superimposed on the endoscope images during the operation together with distance measurements from the tip of the endoscope to the target structure (Fig. 63.4) [23].
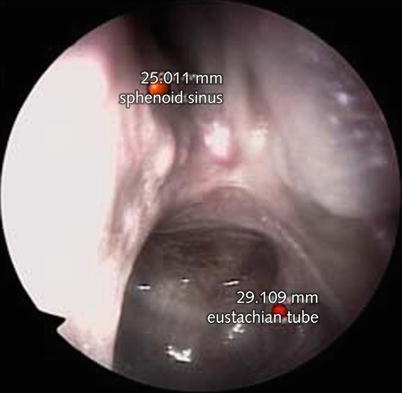

Fig. 63.4
Endoscopic view of the posterior–superior end of the left choana with superimposed marked anatomical points of the location of the natural sphenoid ostium (top) and the posterior lip of the tubarian torus at the entrance to the Eustachian tube as they were defined beforehand in preoperative planning software based on CT imaging. In addition, real-time calculation of distance from the endoscope tip to the target point is calculated and depicted on top of the corresponding red markers (Carversaccio et al., Springer with permission [23])
IGS in Neuro-otology and Lateral Skull Base Procedures
Overview
Navigated surgery of the lateral skull base has its own peculiarities with a surgical field similar to a pyramid with its z-axis pointing to the petrous bone apex, which is longer than the x– and y-axes on the convex base with rather contourless mastoid surface. Matching of the real surface of the patient’s head with a virtual reconstruction of the radiological imaging can be especially challenging [24] as there are less pronounced and prominent surface points compared to the bony and cartilaginous midfacial structures in rhinologic interventions of the anterior skull base. Nevertheless, registration on strategic points such as the tragus, mastoid tip, mastoid foramen, fronto-zygomatic suture, bony ear canal, or even the umbo enables safe and reliable surgery [25] without formerly used invasive rigid registration by screw-fixed fiducials. CAS is much less frequently applied in otologic surgery compared to endoscopic sinus surgery, and evidence-based studies with level of evidence I or II are hardly feasible. Therefore, recommendations [26] for the use of CAS remain moderate (i.e., level of evidence III or IV), albeit individual surgeons who use CAS technology in a larger number of cases subjectively rated CAS as very useful for the surgeon. In sum, possible recommendations for CAS in lateral skull base proceedings endangering vital neurovascular structures, cochleovestibular, as well as cranial nerve function have been updated and proposed (Table 63.2).
Table 63.2
Recommendations for CAS in lateral skull base procedures
1 | Expected anatomical alterations by malformation/trauma |
2 | Mass lesions in the middle or inner ear |
3 | Petrous apex lesions |
4 | Involvement of middle or posterior cranial fossa |
5 | Didactic and training reasons |
Practical Examples
Navigated Neuro-otologic Surgery for Ear Canal Atresia
The main aim of CAS in neuro-otologic proceedings is improved localization and salvage surgery in complex structures of the middle and inner ear, especially if regular anatomical landmarks become highly distorted or are even absent. In cases of various types of ear canal atresia, identification of the optimal drilling site for the creation of a new external ear canal and precise burring respecting the labyrinth block has been enabled in a series of cases by aid of CAS [28]. Moreover, CT data of the temporal bone can be used in a simulator creating a holographic model for the surgeon in order to train and perform strategic surgical steps in a realistic virtual reality (Fig. 63.5).
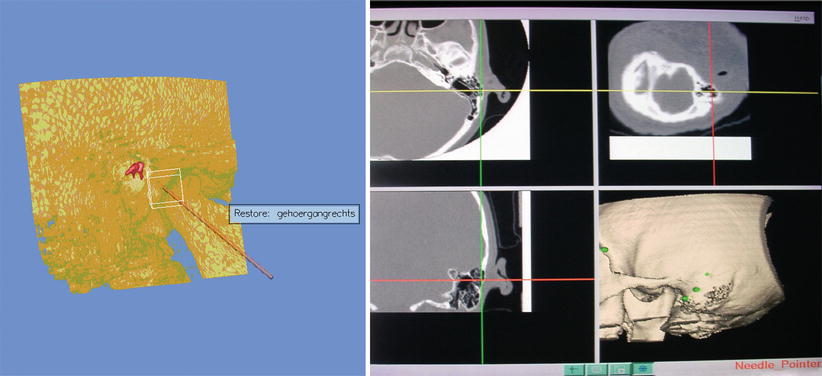

Fig. 63.5
Left Bony ear canal atresia (Schuknecht type C) with planned trajectory for operation on the holographic simulator (Volume Interactions™); Right intraoperative navigation system with crosshair of the burr tip centered in the mastoid (Caversaccio et al., Springer with permission [27])
IGS in Vestibular Schwannoma Removal Limited to the Internal Acoustic Meatus
In a selected number of vestibular schwannoma patients, the tumor is restricted to the inner ear canal and can be accessed extradurally from the middle cranial fossa. Although this approach is surgically elegant, the facial nerve runs in close proximity to the direction of tumor resection, and CAS is extremely useful in predicting and localizing the actual course of the nerve (Figs. 63.6 and 63.7).
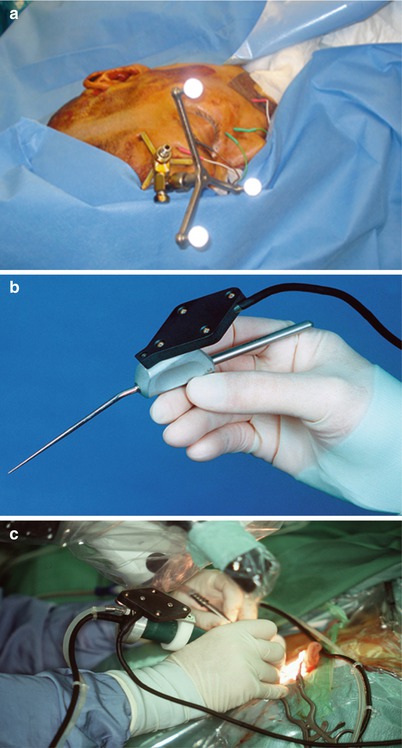
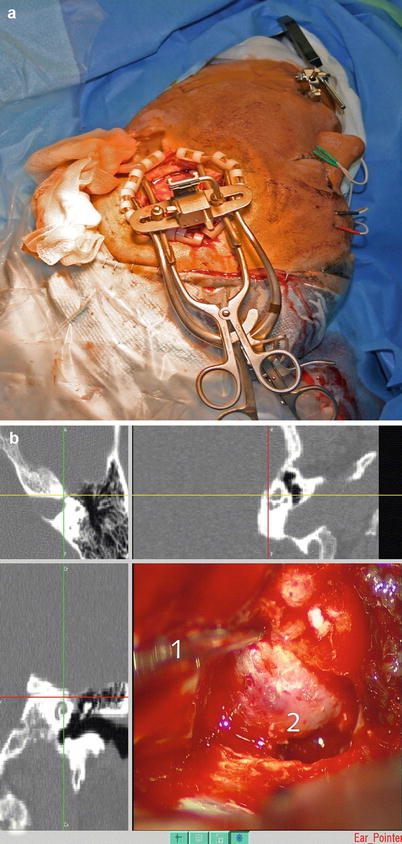

Fig. 63.6
Bone anchored reference star with passive light reflecting spheres (Fa. Brainlab TM, Heimstetten) mounted on the patient’s pteryon (a) and navigated needle pointer (b) and microsurgical drill in action (c)

Fig. 63.7




Transtemporal approach to a Kos type I intrameatal vestibular schwannoma of the left side (a). The intraoperative view depicts the navigated suction tip (1) above the exposed skull base (2) (b) (Source: Caversaccio et al., Springer with permission [27])
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree