and Suzanne K. Freitag3
(1)
Department of Radiology, University of Chicago, 5841 S Maryland Avenue, Chicago, IL 60637, USA
(2)
Head and Neck Imaging, Department of Radiology, University of Chicago, 5841 S Maryland Avenue, Chicago, IL 60637, USA
(3)
Ophthalmic Plastic Surgery Service, Department of Ophthalmology, Massachusetts Eye and Ear Infirmary, Harvard Medical School, Boston, MA, USA
8.1 Overview
A wide variety of benign and malignant neoplasms can affect the orbit, either primarily or secondarily, such as via extension of a tumor from a neighboring structure or as a metastasis. Likewise, there are a multitude of orbital oncological therapeutic modalities available, including surgery, chemotherapy, radiation, cryotherapy, and laser therapy. Diagnostic imaging plays an important role not only in the pretreatment evaluation of orbital region tumors but also for follow-up after treatment in order to monitor tumor response or recurrence. CT and MRI are generally suitable modalities for posttreatment surveillance of the orbit. Positron emission tomography (PET) using [18 F]-2-deoxy-D-glucose (18FDG) serves a role as a staging tool in ophthalmic oncology, particularly for detecting distant metastatic lesions that conventional imaging studies may otherwise miss. Besides the initial workup of orbital tumors, 18FDG-PET can be useful for the assessment of treatment response. Ultrasound with color and spectral Doppler is useful for evaluating small intraocular tumors that are otherwise inconspicuous on CT and MRI. The interpretation of posttreatment imaging can be particularly challenging due to the altered anatomy and tissue properties caused by many forms of treatment. Furthermore, many oncological treatments can cause complications that warrant investigation via diagnostic imaging. Some of these complications have particularly characteristic and interesting imaging findings. In order to optimally approach an imaging study of the orbit after treatment, it is important to know what the original tumor was like, the type and timing of the therapy that was administered, and the clinical status of the patient at the time of the scan. The various types of orbital oncologic treatments and their corresponding expected and complicated imaging features are reviewed in the following sections.
8.2 Intraocular Biopsy
Biopsy is often performed for the diagnosis of orbital masses. Incisional biopsy, which involves sampling a small portion of a lesion, may be useful when a tissue diagnosis is desired, but the lesion is infiltrative and cannot be separated from surrounding structures. Excisional biopsy consists of removing the tumor in its entirety and is both diagnostic and therapeutic. Aside from absence of the lesion, complete excisional biopsy may leave no changes on postoperative imaging. However, there may be perceptible defects in normal anatomic structures that were removed along with the tumor (Fig. 8.1). In addition, there may be alterations in the structures remaining in the region of the excision due to reconstructive efforts. For example, a vascularized nasoseptal mucosal flap used to reconstruct a medial orbital wall defect created during endoscopic transnasal orbitotomy can demonstrate avid enhancement on MRI (Fig. 8.2), potentially mimicking residual tumor. However, the flap has a distinctive linear configuration with a connection to the nasal septum.
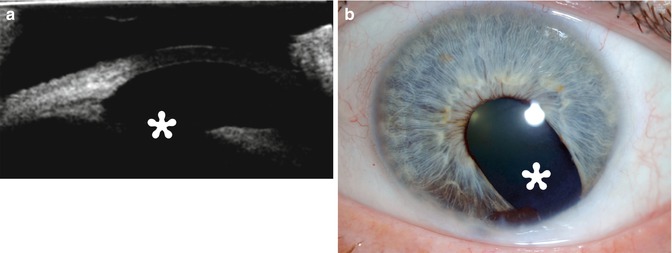
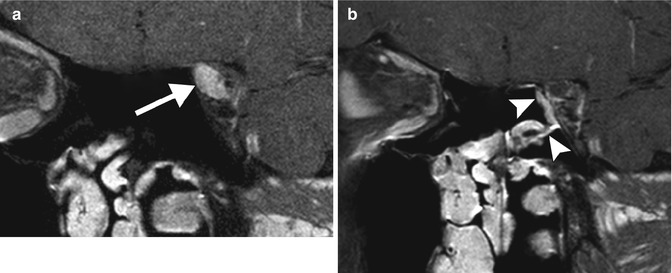

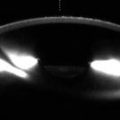
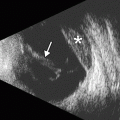
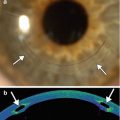
Fig. 8.1
Excisional biopsy of an anterior segment tumor. The patient has a history of iris melanoma excised many years earlier. UBM (a) and corresponding clinical photograph (b) show a defect in the iris and ciliary body (Courtesy of Ivana Kim MD)

Fig. 8.2
Nasoseptal flap. The patient presented with left optic neuropathy due to a cavernous hemangioma in the orbital apex and underwent excisional biopsy via a transnasal endoscopic technique with access through the medial wall of the orbit. Due to the risk of postoperative enophthalmos, the medial wall of the orbit has been reconstructed with a vascularized pedicle flap from the mucosa of the nasal septum. Preoperative coronal post-contrast, fat-suppressed T1-weighted MRI (a) shows a well-defined enhancing lesion in the left orbital apex (arrow). Postoperative coronal post-contrast, fat-suppressed T1-weighted MRI (b) shows linear enhancement along the medial wall of the orbital apex extending from the nasal septum, which corresponds to the transposed mucosal flap (arrowheads). The mucosal flap enhances avidly similar to the preoperative appearance of the tumor. However, there was no evidence of residual tumor (postoperative imaging was obtained to confirm complete tumor resection)
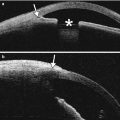
Complications related to orbital biopsy that may lead to imaging evaluation are uncommon and include infection, hemorrhage, visual loss, diplopia, and CSF leak. CT and MRI are both appropriate modalities for evaluating postoperative infection, which can manifest as preseptal and/or postseptal cellulitis, with or without abscess. Hemorrhage can occur anywhere along the path of the biopsy, including within or adjacent to the remaining portions of the biopsied tumor, which can result in transient expansion of the mass. Noncontrast CT and MRI are suitable for the evaluation of post-biopsy hemorrhage, in order to characterize the extent and site of hemorrhage. There can be an acute change in the appearance of the tumor if it has hemorrhaged, which can manifest with fluid-fluid levels on CT or MRI (Fig. 8.3). Disruption of the orbital roof during a biopsy can lead to a CSF leak, which can manifest as an accumulation of fluid within the orbit and pneumocephalus. Thin-section, multiplanar CT without contrast is useful for delineating the defect and planning surgical repair, if necessary (Fig. 8.4). Alternatively, an encephalocele can form through the iatrogenic defect and MRI is useful for characterizing this entity prior to surgical repair.
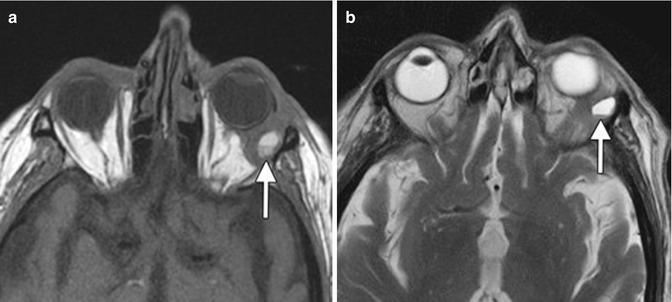
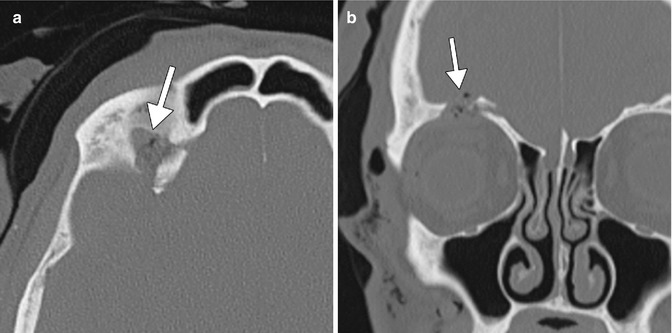

Fig. 8.3
Post-biopsy hemorrhage. The patient underwent recent left anterolateral orbitotomy for biopsy of a left orbital tumor. Axial T1-weighted (a) and T2-weighted (b) MR images show a fluid-fluid level within the left orbital mass (arrows)

Fig. 8.4
Post-biopsy CSF leak. The patient underwent recent orbital tumor biopsy. Axial (a) and coronal (b) CT images show a defect in the right orbital roof and a small amount of pneumocephalus (arrows). The biopsy was performed to evaluate for perineural tumor spread involving the supraorbital nerve
8.3 Enucleation
Enucleation refers to removal of the eye. The main oncological indications for enucleation include uveal melanoma, retinoblastoma, or other less common intraocular tumors that are large, recurrent, or poorly responsive to treatment. The extraocular muscles are disinserted from the globe prior to its removal from the socket. The muscles may be reattached to the orbital implant in a variety of ways, including suturing muscle to muscle in front of the implant, suturing the muscle directly to the implant, or suturing the muscle to the material used to wrap the implant. The void created by removing the globe is typically filled with an orbital implant (Fig. 8.5). The most commonly used implants are composed of silicone, hydroxyapatite, and porous polyethylene (Medpor). The imaging features of the various implant materials are discussed and depicted in extensive detail in Chap. 5. However, notably, certain implants, such as Medpor, can demonstrate internal enhancement due to fibrovascular ingrowth, which should not be mistaken for tumor involvement (Fig. 8.6). Furthermore, in the early postoperative period, enhancement and T2 hyperintensity of the orbital tissues are expected findings (Fig. 8.7) and may represent granulation tissue or a transient inflammation. Although this is a characteristically diffuse process, it can be difficult to discern an underlying tumor, and follow-up imaging is recommended if there is any doubt. Despite the potential artifacts, MRI with contrast is generally the best modality for surveillance imaging after enucleation for retinoblastoma in order to delineate local and regional tumor recurrence, trilateral retinoblastoma, as well as distant metastases to the spine. In addition, intraocular melanoma has a particular propensity to metastasize to the liver (95 % of metastases) and occasionally to the brain (Fig. 8.8). Contrast-enhanced CT and 18FDG-PET are also appropriate imaging modalities for metastatic workup in the bones, chest, and abdomen, depending upon the type of primary tumor and location of the suspected metastasis. For example, melanoma and squamous cell carcinoma tend to be markedly hypermetabolic on 18FDG-PET, unless there is considerable necrosis, while adenoid cystic carcinoma tends to not be so conspicuous. A potential pitfall on 18FDG-PET is the presence of orbital implants. While silicone implants do not appear hypermetabolic on 18FDG-PET (Fig. 8.9), Medpor and hydroxyapatite implants with fibrovascular ingrowth can appear diffusely hypermetabolic (Fig. 8.10) due to physiologic activity. This should not be mistaken for neoplasm or infection, which tends to display a greater degree of hyperpigmentation in components beyond the implant itself.
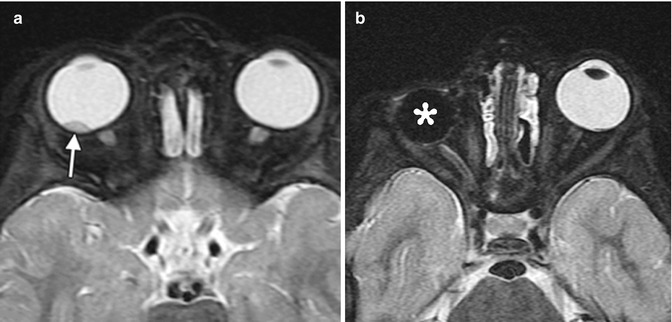
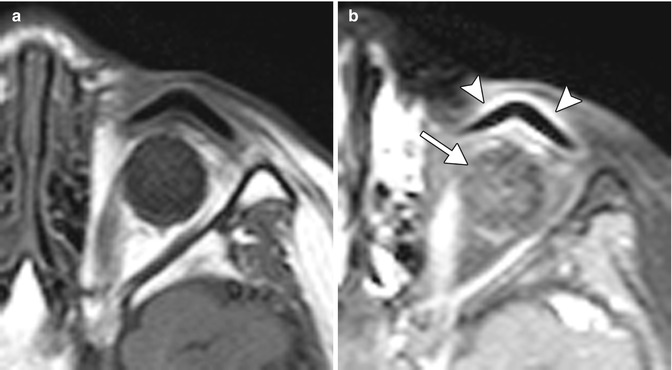
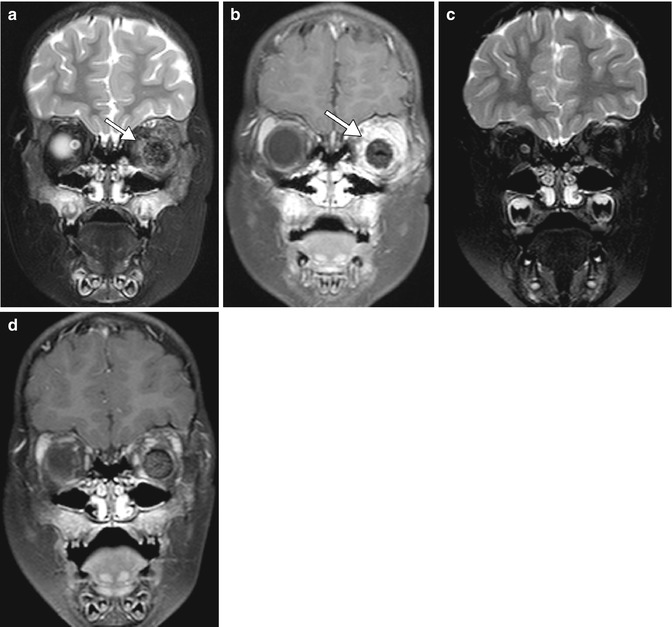
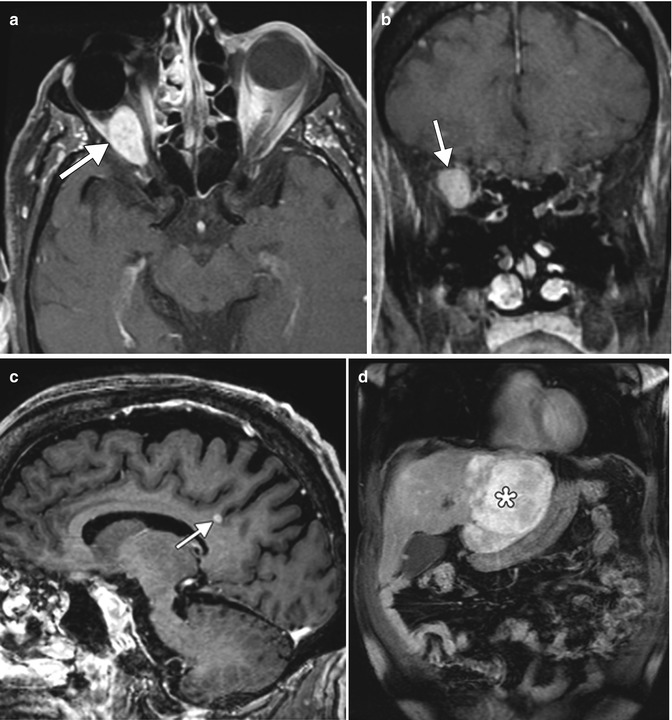
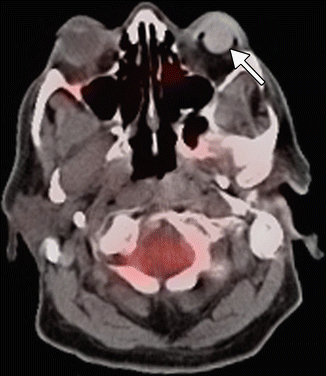
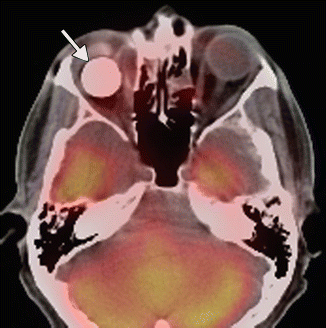

Fig. 8.5
Enucleation. Preoperative axial fat-suppressed T2-weighted MRI (a) shows a right intraocular mass, consistent with retinoblastoma (arrow). Postoperative axial fat-suppressed T2-weighted MRI (b) shows interval removal of the right globe and insertion of a silicone implant. Much of the right optic nerve remains

Fig. 8.6
Porous polyethylene (Medpor) implant fibrovascular ingrowth and ocular prosthesis artifact. Axial T1-weighted (a) and axial post-contrast fat-suppressed T1-weighted (b) MR images show expected diffuse enhancement of the Medpor implant (arrow). There is also linear enhancement of the conjunctiva surrounding the ocular prosthesis (arrowheads)

Fig. 8.7
Expected early postoperative findings related to enucleation. Initial postoperative coronal fat-suppressed T2-weighted (a) and post-contrast fat-suppressed T1-weighted (b) MR images show diffuse T2 hyperintensity and enhancement of the orbit surrounding the implant (arrows). The corresponding follow-up MR images obtained 6 months later (c, d) show near interval resolution of these findings, although there is persistent mild enhancement and swelling of the extraocular muscles. A Medpor implant is present

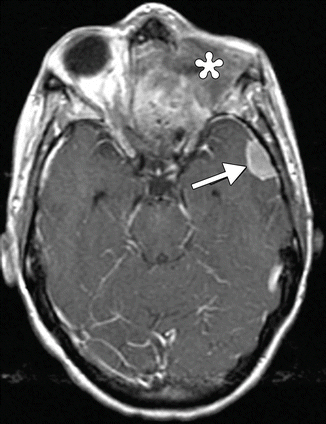
Fig. 8.8
Recurrent choroidal melanoma with metastases. Post-contrast fat-suppressed axial (a) and coronal (b) T1-weighted MR images show an enhancing mass in the right orbit, posterior to the ocular implant. Sagittal post-contrast T1-weighted MRI (c) shows an enhancing metastasis in the brain (arrow). Coronal post-contrast T1-weighted MRI (d) of the abdomen shows a large enhancing metastasis within the liver

Fig. 8.9
Silicone orbital implant on positron emission tomography/CT imaging. The 18FDG-PET/CT fusion image shows absence of hypermetabolism in the left orbital prosthesis (arrow)

Fig. 8.10
Hydroxyapatite implant on positron emission tomography/CT. The 18FDG-PET/CT fusion image shows mild diffuse hypermetabolism within the right orbital hydroxyapatite prosthesis (arrow). There were no symptoms related to the implant and there was no evidence of tumor in the orbits
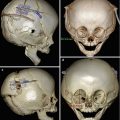
Besides surveillance of tumor recurrence and metastases, diagnostic imaging is also useful for evaluating complications related to enucleation, which are depicted in Chap. 5. However, enucleation during infancy due to retinoblastoma or other causes can lead to the development of microorbitalism due to the decreased growth stimulation from the globe and surrounding soft tissues (orbital arrest syndrome). The ipsilateral maxillary sinus is frequently hypoplastic as well. High-resolution multiplanar facial bone CT imaging (Fig. 8.11) is useful for characterizing the abnormalities and for corrective surgical planning, which may consist of osteotomy and expansion of the orbital vault.
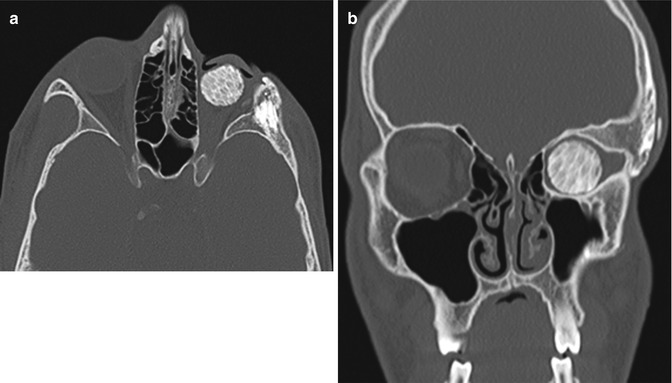

Fig. 8.11
Orbital arrest syndrome (microorbitalism). Axial (a) and coronal (b) CT images show a relatively small left orbital cavity, which contains a hydroxyapatite implant. In particular, there is sclerosis and thickening of portions of the left orbital walls. There is also hypoplasia of the left maxillary sinus, which is depicted on the coronal image
8.4 Exenteration
Exenteration involves removal of the orbital contents, including the globe and varying amounts of periorbital tissue. Exenteration is a radically disfiguring procedure that is most often reserved for the treatment of life-threatening malignancies arising from the orbit, paranasal sinuses, or periocular skin. The most common diagnoses requiring orbital exenteration include basal cell carcinoma, sebaceous carcinoma, squamous cell carcinoma, melanoma, and lacrimal gland malignancies. There are various classifications of exenteration surgery based on which tissues are removed. For example, Meyer and Zaoli’s classification describes four types of exenteration. In type I, the palpebral skin and conjunctiva are spared. In type II, only the palpebral skin is spared and the globe and its appendages are removed with the conjunctiva. In type III, the eyelid is removed with the orbital contents. In type IV, the globe, eyelids, and appendages of the eye are removed with the involved bony structures. Various methods for reconstruction of the exenteration cavity can be implemented, including partial- or full-thickness skin graft, dermis fat grafts, free or pedicled musculocutaneous flaps, and artificial implants (Figs. 8.12, 8.13, and 8.14). Of note, on postoperative MRI or CT imaging, the viable muscular portion of myocutaneous flaps normally displays enhancement, which should not be mistaken for tumor recurrence. Patients may wear an eye patch following surgery or have an ectoprosthesis that features the globe and eyelids, which are custom fit for cosmetic purposes. The prostheses can be made from a variety of materials, such as polymethyl methacrylate and silicone. These prostheses are retained with adhesives, tissue undercuts, or magnets once the socket has healed. The prosthesis is not always removed during diagnostic imaging exams of the region, which usually does not pose an issue with regard to artifacts since the majority of these do not contain metallic components and the underlying exenteration cavity can be readily delineated (Fig. 8.15).
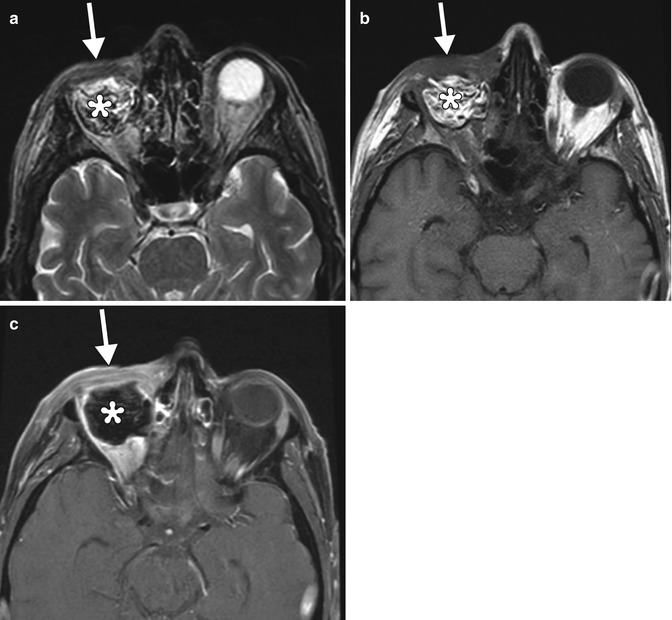
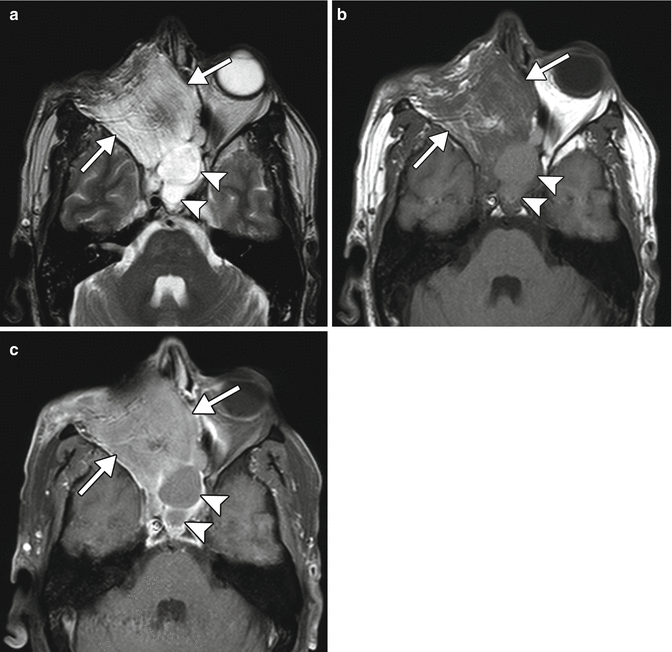
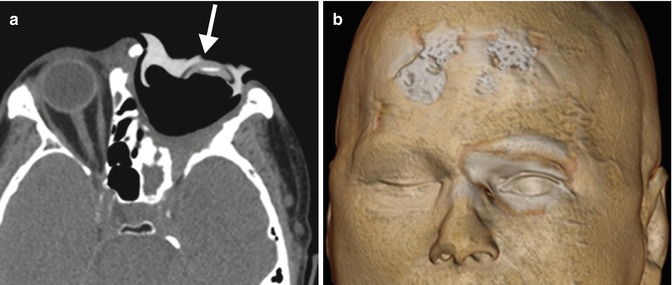
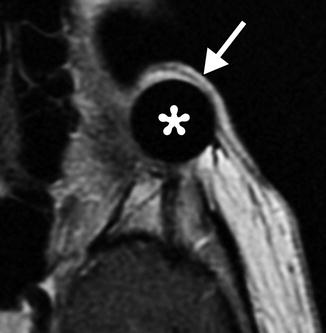

Fig. 8.12
Eyelid-sparing exenteration with fat graft reconstruction. The patient had a history of recurrent choroidal melanoma after enucleation. Axial T2-weighted (a), T1-weighted (b), and post-contrast fat-suppressed T1-weighted (c) images show preservation of the eyelids (arrows), which are mildly swollen and enhancing due to recent surgery. Fat graft is present within the exenteration cavity

Fig. 8.13
Orbital exenteration and maxillectomy with myocutaneous graft reconstruction. The patient has a history of a salivary gland malignancy involving the right orbit. Axial T2-weighted (a), T1-weighted (b), and fat-suppressed, post-contrast T1-weighted (c) MR images show striations and enhancement of the muscular component of the flap (arrow) and trapped secretions in the sphenoid sinuses (arrowheads)

Fig. 8.14
Orbital exenteration with cosmetic ectoprosthesis. The patient has a history of basal cell carcinoma involving the left face with orbital invasion and is status post orbital exenteration. Axial (a) CT image shows a custom-fit hyperattenuating prosthesis (arrow) that covers the left orbital exenteration cavity, which is lined by a skin graft. The 3D volume-rendered CT image (b) shows a satisfactory cosmetic result, although the artificial eyelids do not blink

Fig. 8.15
Exenteration with implant. The patient has a history of left choroidal melanoma with extrascleral extension, status post left orbital exenteration with augmentation of the cavity with a spherical silicone implant affixed to the lateral orbital rim, as shown on the axial T1-weighted MRI, in which the hypointense implant is covered by skin graft (arrow)
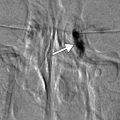
Complications have been reported in over 20 % of exenterations in the setting of orbital oncology and include fistula formation, infection, flap necrosis, CSF leak, and tumor recurrence. Fistulas most commonly occur from the orbit to the ethmoid sinuses but can extend to other sites. On imaging, fistulas may appear as air-filled channels, which are best depicted on CT (Fig. 8.16). Fistulas are sometimes associated with infection. Otherwise, infections appear as nonspecific fluid collections that can be difficult to distinguish from seromas or CSF leaks based on imaging alone. Tumor recurrence most commonly occurs at the margins of the reconstruction flap (Fig. 8.17). It is also important to be vigilant for lymph node metastases, intracranial metastases, and perineural tumor spread, all of which can manifest after exenteration. CT with contrast is typically adequate for delineating lymph nodes, while MRI with contrast is more sensitive for depicting intracranial metastases. MRI with contrast is also particularly useful for depicting the presence of perineural tumor spread, which appears as excess thickening and enhancement of the involved nerve segments (Fig. 8.18). The incidence and form of tumor recurrence are highly dependent on the tumor histology and staging. For example, squamous cell carcinomas and adenoid cystic carcinomas have a relative propensity for perineural spread. In addition, adenoid cystic carcinomas can metastasize to the dura and mimic meningiomas (Fig. 8.19).
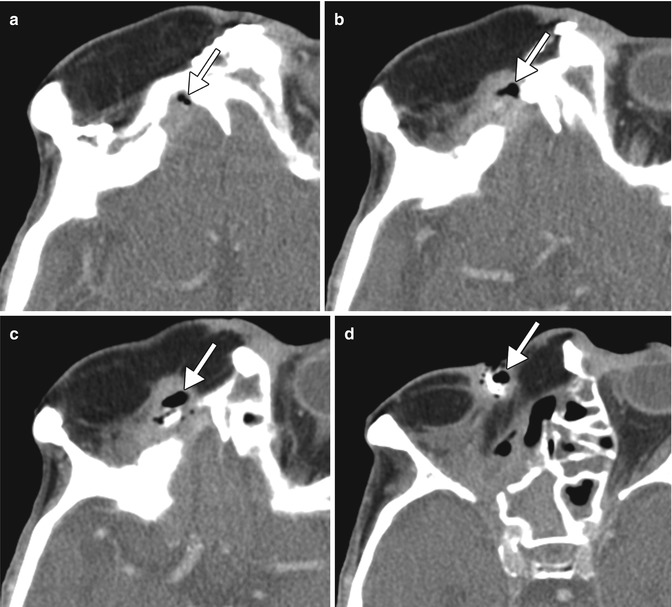
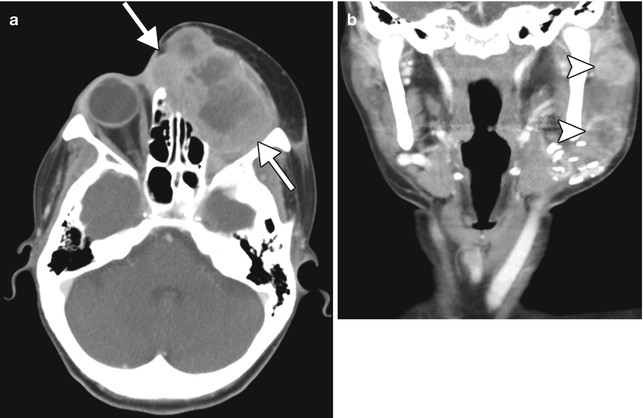
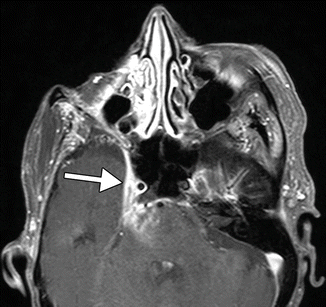

Fig. 8.16
Fistula. The patient underwent orbital exenteration with removal of a portion of the orbital roof and paranasal sinuses. Axial CT images (a–d) show an air-filled tract (arrows) that extends from the epidural space adjacent to an area of bony dehiscence to a skin defect along margins of the myocutaneous flap

Fig. 8.17
Local and regional tumor recurrence. The patient has a history of squamous cell carcinoma that involved the orbit, which was treated with orbital exenteration and myocutaneous flap reconstruction. Axial CT image (a) shows a heterogeneously enhancing mass (arrows) that occupies the exenteration bed, deep to the flap. In addition, the coronal CT image (b) shows metastatic lymphadenopathy (arrowheads)

Fig. 8.18
Perineural tumor spread. The patient has a history of facial squamous cell carcinoma with invasion of the right orbit and is status post orbital exenteration. Axial post-contrast fat-suppressed T1-weighted MRI shows diffuse thickening and enhancement of the right trigeminal nerve with extension into the pons (arrow)