div class=”ChapterContextInformation”>
8. Current Imaging Approaches and Challenges in the Assessment of Peripheral Artery Disease
Keywords
Acute limb ischemiaAngiographyCatheter angiographyComputed tomography angiography (CTA)Digital subtraction angiography (DSA)Doppler ultrasoundGadolinium-based contrast agents (GBCAs)Gadolinium depositionIodinated contrastIonizing radiationMagnetic resonance angiography (MRA)Magnetic resonance imaging (MRI)Nephrogenic systemic fibrosis (NSF)Peripheral arterial disease (PAD)X-ray angiography (XA)Introduction
The current standard of care for imaging peripheral artery wall pathology predominantly focuses on quantifying the vascular lumen size using digital subtraction angiography (DSA), computed tomography angiography (CTA), and magnetic resonance angiography (MRA). In larger, central vessels, magnetic resonance can also be used to measure velocity and volume of flow. Velocity of blood flow can also be measured using Doppler ultrasound (US), in addition to visualizing vessel size with grayscale US; however, vascular wall calcifications create artifacts that limit visualization of intraluminal flow. US is highly user dependent, and even when a skilled technician is available, the examination of the arterial system of an extremity is time-consuming. Interestingly, calcium possibly poses the greatest challenge in visualizing extremity vessel walls and lumens, as it may result in imaging artifacts in other modalities also.
This chapter will review standard peripheral artery imaging paradigms, focusing on peripheral arterial disease and acute limb ischemia, the most common and emergent peripheral vessel wall diseases in the United States, respectively. Conventional standard imaging paradigms and their benefits and limitations will be discussed, predominantly in the context of these common conditions.
Peripheral Arterial Disease and Acute Limb Ischemia
Background
Approximately 8.5 million Americans (7.2%) age 40 years or older are estimated to have peripheral arterial disease (PAD), defined as a low ankle-brachial index (<0.9) in 6.5 million and ABI > 0.9 after revascularization therapy or false-negative ABI > 0.9 in an additional 2 million [1, 2]. Based on 2003–2008 nationwide ICD coding from large employers, Medicare and Medicaid, the incidence of PAD in adults over 40 years old was over 12% [2, 3]. At least 1.6 million (approximately 25% of patients with low ABI) of these are estimated to have severe PAD, with ABI < 0.7 [1, 2]. Approximately 1.3% of American adults over 40 years of age have critical limb ischemia [2, 3], the most severe presentation of PAD.
The etiology of peripheral arterial disease is atherosclerosis, due to hardening and thickening of arterial walls. This process is exacerbated by smoking, diabetes mellitus, and hypertension, especially in the arterioles. Ultimately vessel lumens narrow so that blood flow becomes turbulent. Emboli may acutely and completely obstruct already narrowed arteries. The most severe presentation is acute limb ischemia (ALI), also known as a cold limb, when a vessel lumen is sufficiently obstructed so that blood flow approaches zero.
Acute limb ischemia is most commonly caused by complete arterial occlusion [4] associated with PAD; however, there are other causes. In a young and otherwise healthy person, cystic adventitial disease may cause ALI with markedly decreased or absent peripheral pulses. Trauma and prolonged exposure are usually clinically obvious. In traumatic posterior dislocation of the knee, the popliteal artery and distal pulses must be carefully evaluated. Patients with total venous outflow occlusion, an uncommon cause of ALI, usually present with the precursor to venous gangrene, known as “phlegmasia cerulea dolens,” or swollen, dusky blue, and painful extremity; the presence of peripheral pulses readily excludes arterial occlusion as the etiology [4].
Ankle-Brachial Index and Ultrasound
Ankle-brachial index, toe brachial index, and handheld Doppler can be used at the bedside to support a diagnosis of ALI [4]. Thermographic (infrared camera) attachments for multiple brands of smartphones are commercially available to the general public, for the cost of approximately US $200.00, and provide another option for noninvasive bedside evaluation of distal extremity perfusion [5]. Although all of the bedside evaluations in this paragraph may aid in prompt clinical diagnosis of ALI, they are all insufficient for arterial mapping and interventional planning. Despite its wide availability, the role of ultrasound and Doppler is limited in ALI for several reasons. Even when a skilled user is available, the exam of the arterial system of an extremity is rather time-consuming. Also vascular calcifications will prevent visualization of intraluminal flow due to artifact.
Catheter Angiography
When there is high clinical suspicion for ALI, catheter angiography, also known as x-ray angiography (XA) and digital subtraction angiography (DSA) which are used interchangeably throughout this chapter, remains the gold standard, because of its high resolution and because diagnosis and treatment are possible in the same procedure. The American College of Radiology (ACR) recommends DSA when there is an intermediate level of suspicion of an acutely obstructed artery [4]. Despite its widespread availability, XA poses multiple risks to the patient. There is the possibility of iodinated contrast injury to the kidneys, which can be decreased by patient selection. The invasive nature of XA, even when the diagnostic portion of the procedure ultimately demonstrates no need for interventional treatment, poses the risk of numerous complications, including but not limited to hemorrhage, pseudoaneurysm, dissection, subcutaneous hematoma, and infection. Additionally, exposure to ionizing radiation poses both risks of deterministic injury and increased risk of cancer over the patient’s lifetime.
Computed Tomography Angiography
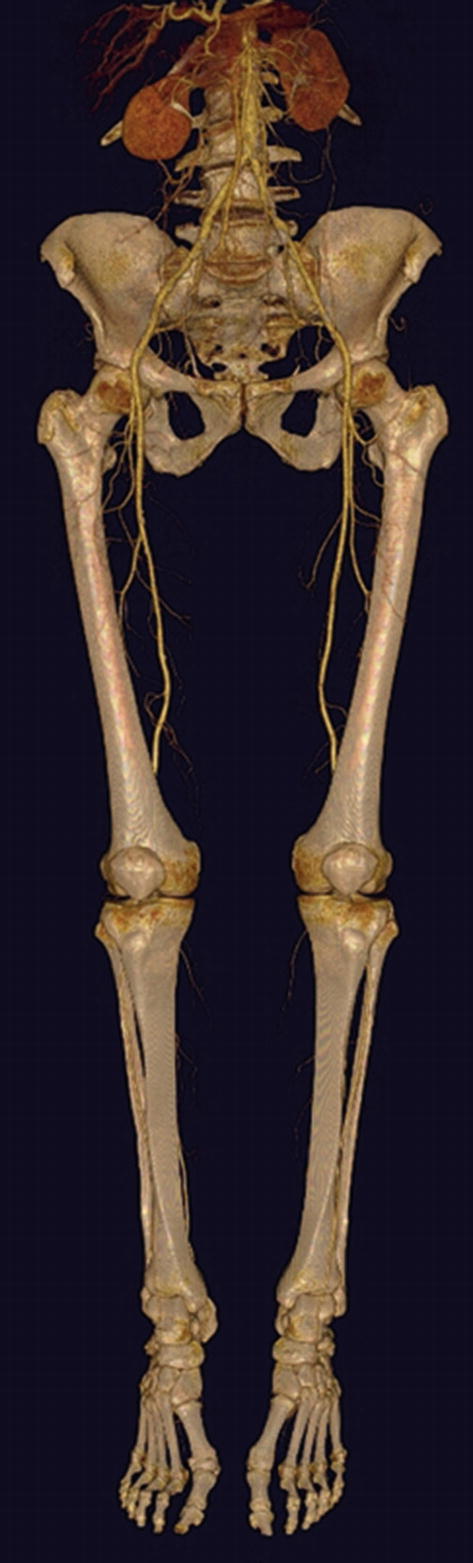
CTA runoff including abdominal aorta, pelvic arteries, and arteries in the lower extremities can typically be performed in less than 20 seconds of scan time
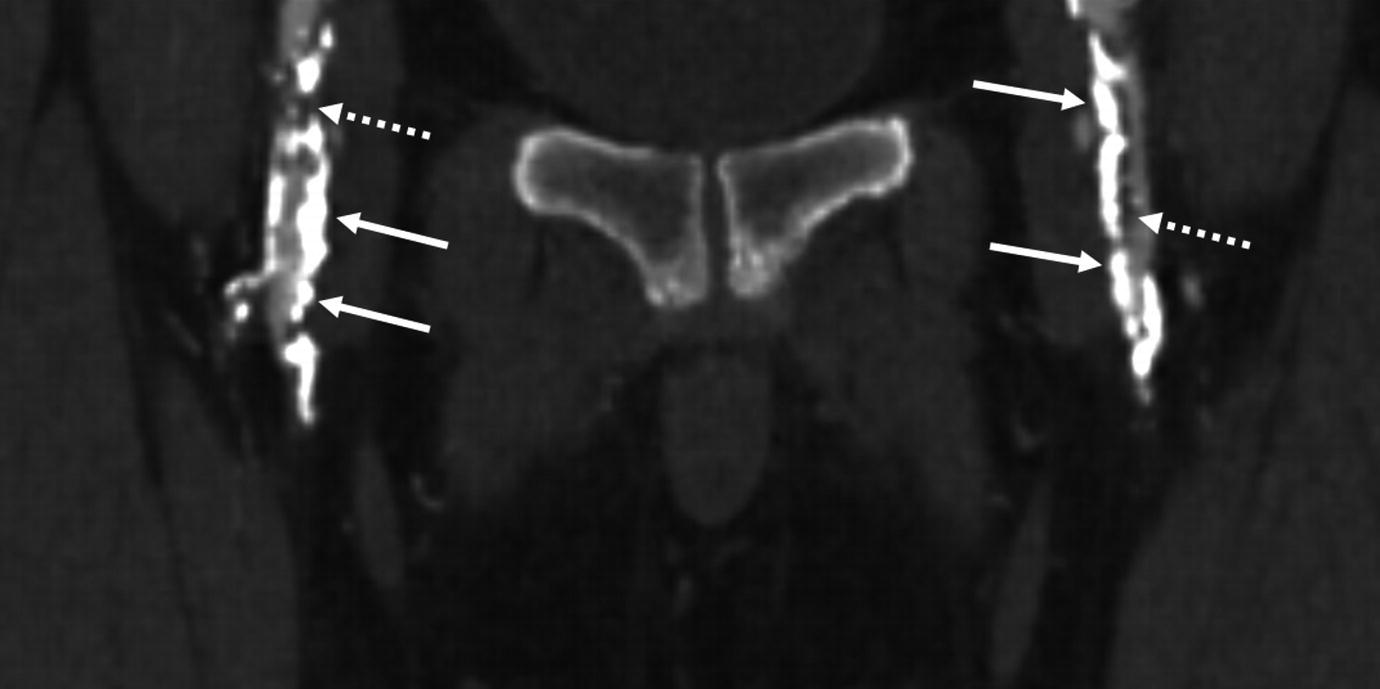
Calcified plaque can limit the diagnostic quality of CTA. Calcified plaques (solid arrows) in the superficial femoral arteries appear to cause severe bilateral stenosis. Non-calcified plaque (dashed arrows) do not cause blooming artifact
Magnetic Resonance Angiography
Appropriate catheters and guidewires for magnetic resonance image-guided intravascular procedures are under development [6, 7]; however until these are widely available, magnetic resonance imaging will continue to be used predominantly for diagnostic imaging in PAD and ALI. Magnetic resonance angiography (MRA) without and with IV contrast in suspected limb ischemia is considered usually appropriate, according to the ACR appropriateness criteria [4].
In a 2013 meta-analysis, no significant difference in sensitivity or specificity was found when comparing CTA to contrast-enhanced (CE) MRA [8]; this was especially true when dedicated additional CE MRA imaging of the calf was performed. In the acute setting, however, the use of MRA may be restricted by the lack of availability of an MR technician and/or MRI table time. Assuming a technician and the magnet itself are available, radiologists may consider non-contrast-enhanced MRA as well as a post-contrast T1-weighted MRA. T2-weighted magnetic resonance imaging without, or prior to, contrast would demonstrate some secondary signs of acute limb ischemia, such as soft tissue edema.
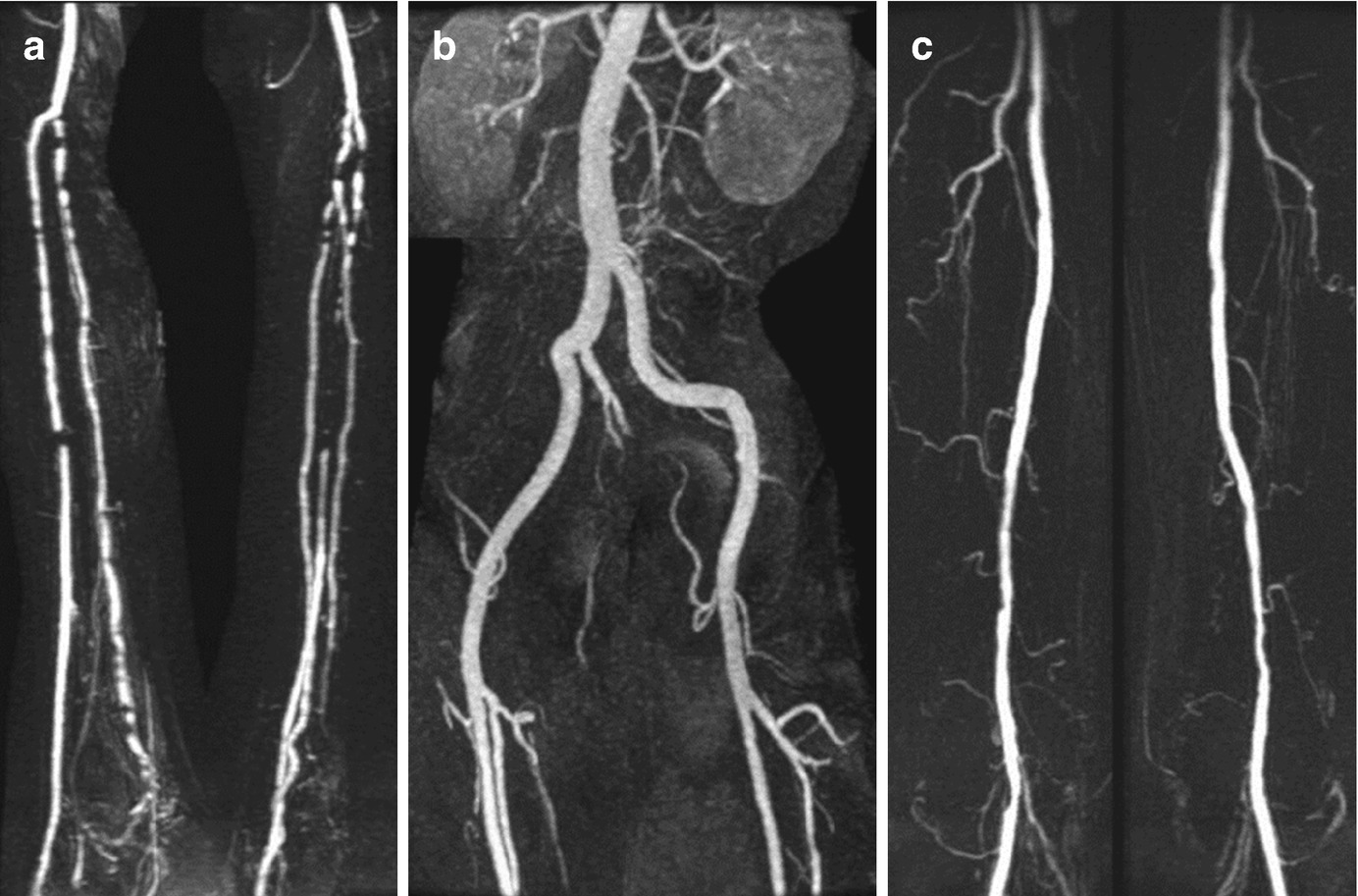
Hybrid peripheral MRA approach consists of (a) time-resolved contrast-enhanced (CE) MRA in the calves followed by (b) 3D CE MRA in the pelvis and (c) 3D CE MRA in the thighs
The ACR notes that MRA without IV contrast is “sometimes appropriate,” particularly in patients with estimated glomerular filtration rate (eGFR) of <30 mL/min [4]. However, no unconfounded case of nephrogenic systemic fibrosis (NSF) was found in over 1400 high-risk inpatients who received gadobenate dimeglumine (MultiHance) [14]. Also, there was no case of NSF in 401 patients with decreased renal function, 303 of whom were dependent on dialysis, after administration of gadobenate dimeglumine [15]. Since 2008, only one case of NSF has been reported with any gadolinium-based contrast agent, and this was in a patient with normal eGFR [16]. American College of Radiology classifies gadolinium-based contrast agents (GBCAs) as follows: Group I GBCAs are associated with the greatest number of NSF cases; Group II GBCAs are associated with few, if any, unconfounded cases of NSF; and Group III GBCAs have limited data however are also associated with few, if any, unconfounded cases of NSF [17].
Concern for nephrogenic systemic fibrosis (NSF) after gadolinium-based contrast administration heightened interest in non-contrast MRA sequences. Non-contrast-enhanced (NE) MRA eliminates the exceedingly low possibility of NSF; however, our institution has identified zero cases of NSF since gadodiamide was removed from the gadolinium-based contrast agent (GBCA) formulary [18]. NE MRA also eliminates gadolinium retention and any as-of-yet-unknown associated risks [19, 20]. Patient preference may be to avoid GBCAs due to recent lay press coverage of gadolinium deposition and new symptoms following GBCA exposure, the US Food and Drug Administration issuance of a new class warning regarding the occurrence of gadolinium deposition, and the European Medicines Agency recommending suspension of use of some GBCAs [21, 22]. NE MRA has greatly improved in the last decade; however, challenges still remain.
The limitations of NE MRA sequences commercially available in 2011 were well summarized by Hodnett et al. [23]. Gated 2D time-of-flight imaging requires excessive scan time and results in less than ideal image quality often due to artifacts attributed to in-plane flow. Subtraction techniques are unfortunately highly sensitive to patient motion and require precise timing of systolic imaging during the peak velocity of blood flow for the vessel segment being images, “which is both patient- and operator-dependent” [23]. Electrocardiogram (ECG)-gated 3D half-Fourier fast spin echo (FSE) is one such subtraction technique, with which Lim et al. were technically successful just over half the time [24, 25]. Fan et al. explored flow-sensitive dephasing (FSD)-prepared balanced steady-state free precession (SSFP); however this also requires long imaging time and image subtraction, both of which increase sensitivity to motion artifacts [26].
As described in multiple 2011 publications, Hodnett et al. explored the use of quiescent-interval single-shot (QISS) MRA, which was not commercially available at the time [23, 27, 28]. QISS NE MRA at 1.5 Tesla has demonstrated high diagnostic accuracy compared with DSA and improved visualization of heavily calcified arteries in the lower extremities when compared to CTA [29]. In 3 Tesla magnetic fields, NE QISS MRA demonstrated similar sensitivity and specificity to CTA overall; however, NE QISS MRA sensitivity was significantly higher than CTA in heavily calcified segments [30]. These various MR sequences are described in more detail below.
In 2011, QISS, MRA images of the arteries from the aortic bifurcation to the feet were obtained in approximately 6 minutes. These two-dimensional images were obtained sequentially via ECG-gated balanced steady-state free precession acquisition [27]. Each thin-slice two-dimensional image is obtained during diastole, after electromagnetic pulses eliminate signal from soft tissue in the slice as well as from anticipated venous inflow. Imaged arterial signal intensity thus depends only upon arterial inflow time, making heart rate and arrhythmias essentially irrelevant. Hodnett et al. found this technique interchangeable with MRA with contrast and with DSA due to similar results for all three modalities attributed to short table time, motion insensitivity, and lack of adjustments required for each patient [23, 27].
Liu et al. provide an excellent summary of the current challenges facing the three main non-contrast-enhanced (NE) MRA techniques: quiescent-interval single-shot (QISS), described above; flow-sensitive dephasing (FSD)-prepared balanced steady-state free precession (SSFP), referred to simply as FSD SSFP; and non-contrast angiography of the arteries and veins sampling perfection with application-optimized contrast by using different flip angle evaluation, called [31].
Ward et al. compared QISS and NATIVE SPACE to contrast-enhanced MRA, time-resolved in the calf only, at 1.5 Tesla, and found QISS to have greater specificity and image quality than NATIVE SPACE [32]. Also, poor quality of multiple NATIVE SPACE images resulted in two of two reading radiologists to incorrectly identify segmental occlusion which was not present on QISS or contrast-enhanced MRA. CE MRA technique included “time-resolved coronal fast low angle shot-based sequence (TWIST – time-resolved angiography with interleaved stochastic trajectories) …[and] stepping table MRA for the remainder of the peripheral vascular system” [32].
Hansmann compared QISS at 3 T to contrast-enhanced MRA and also to digital subtraction angiography [33]. Similar to Ward’s methodology, CE MRA was obtained with time-resolution only in the calf. Due to time-consuming shimming, QISS acquisition time at 3 T was markedly increased to approximately 18 minutes. Motion artifact was attributed to this increased imaging time. QISS imaging was as sensitive to stenosis greater than 50% as contrast-enhanced MRA, however, and in several instances better correlated with DSA.
Zhang et al. [34] compared NE MRA to contrast-enhanced CE MRA obtained at 3.0 T magnetic field strength. NE MRA images were obtained using a flow-sensitive dephasing (FSD) technique that depends on magnitude subtraction of two images obtained sequentially via ECG-gated, 3D balanced SSFP acquisition. Dark artery measurements were obtained during systole and bright artery measurement obtained during diastole, when relatively slow arterial flow results in what is similar to a high T2 signal intensity. The images were obtained over 4–5 minutes. Magnitude subtraction of the two images results in easy visualization of the arteries, without venous contamination and without much visualization of the soft tissues. Aside from the relatively longer table time, dark artery image dependence on univector arterial flow velocity resulted in artifact resembling stenosis in vessel segments oriented transversely. Contrast-enhanced MRA images were obtained using 3D gradient-echo (fast low-angle shot, or FLASH) sequence in three coronal images both prior to and following intravenous contrast administration after contrast arrived in the abdominal aorta as measured using a 2D gradient-echo sequence. These images were obtained in approximately 1 minute; however, they were not time-resolved and were found to have venous contamination. Non-contrast-enhanced MRA overestimated stenoses in comparison to contrast MRA.
Liu et al. compared imaging of calf vessels using non-contrast-enhanced (NE) MRA also using FSD 3D SSFP acquisition at 1.5 T to contrast-enhanced (CE) MRA [35] and at 3.0 T to CE MRA as well as DSA [31]. In both studies, CE MRA were obtained using the same sequences as Zhang et al. [34], again which were not time-resolved. Similarly, NE MRA overestimated stenoses compared to CE MRA, and similarly, venous contamination was sometimes problematic in CE MRA images.
Zhang et al. [36] also compared two NE MRA sequences of the calf arteries, FSD SSFP and QISS, with contrast-enhanced MRA at 1.5 T. ECG-gated QISS required about 4-minute acquisition time, only slightly less than FSD SSFP acquisition time. FSD SSFP had slightly higher specificity than QISS, but otherwise the two NE MRA sequences had similar sensitivity and negative predictive value for stenosis greater than 50%. CE MRA was approximately 1-minute acquisition time.
In summary, although much progress has been made in non-contrast-enhanced MRA of the peripheral arteries, at present we continue to favor MRA with contrast over non-contrast-enhanced MRA FSD SSFP sequence, due to decreased time of acquisition, decreased motion-artifact sensitivity, and lack of heart rate and arrhythmia dependence. The addition of time-resolution contrast-enhanced MRA would reduce or eliminate venous contamination noted in contrast-enhanced MRA obtained using 3D gradient-echo (fast low-angle shot, FLASH) sequence.
The Challenges Facing Current Imaging Paradigms
Like any medical test, ideally every imaging test ordered would be appropriate to the relevant diagnostic question, after a thorough patient interview and physical examination. The ideal imaging test would minimize risk to the patients – this would require it to be noninvasive and expose a patient to minimal or no ionizing radiation. The test would be reliable with rapid and consistent results.
In peripheral arterial disease, an ideal imaging test would clearly demonstrate vessel wall thickness, vessel wall elasticity, and direction, rate, and volume of intraluminal flow. Also, improved means of visualizing the perfusion of adjacent tissue would be helpful to clinical decision-making. Finally, an ideal test would provide information that enables us to predict future progression of disease, even in an asymptomatic patient.
In peripheral arterial disease, we currently prefer MRA without and with contrast, including time-resolved imaging. CTA with iodinated contrast remains a reliable and fast diagnostic option; however, this poses increased risk to the kidneys and ionizing radiation exposure relative to contrast-enhanced MRA. There is ongoing improvement of non-contrast-enhanced MRA for clinical evaluation of peripheral arterial disease. Techniques for evaluating microvasculature in peripheral arterial disease that are not yet commonly used in the clinical setting include contrast-enhanced ultrasound, contrast-enhanced MRI perfusion imaging, non-contrast-enhanced blood-oxygen-level-dependent (BOLD) and arterial spin labeling (ASL) MRI, and magnetic resonance spectroscopy with 31-phosphorus [37, 38].
