Imaging plays a central role in the evaluation of patients following cardiothoracic surgery, both for monitoring in the early postoperative period and for assessing for suspected complications. Patients with postsurgical complications can develop a range of signs and symptoms, from hypotension and tachycardia, as the result of severe bleeding, to fever and leukocytosis because of infection. The radiologist is an important member of the care team in the postoperative period, helping identify and manage complications of cardiothoracic surgery. This article reviews the common complications of cardiothoracic surgery focusing on the role of imaging and clues to diagnosis.
Key points
- •
The radiologist is an important contributor to the postoperative care of patients undergoing cardiothoracic surgery.
- •
Early identification of postoperative complications has the potential to reduce morbidity and mortality.
- •
Chest imaging can identify postsurgical complications of cardiothoracic surgery before they are clinically apparent and confirm their presence and further characterize them when they are suspected.
- •
Knowledge of the spectrum of complications following cardiothoracic surgery and of their respective imaging manifestations is essential for the radiologist to make a meaningful impact on patient care.
Introduction
Imaging plays a central role in the evaluation of patients following cardiothoracic surgery, both for monitoring in the early postoperative period and for assessing for suspected complications. Patients with postsurgical complications can develop a range of signs and symptoms, from hypotension and tachycardia as the result of severe bleeding to fever and leukocytosis because of infection. The radiologist is an important member of the care team in the postoperative period, helping identify and manage complications of cardiothoracic surgery. This article reviews the common complications of cardiothoracic surgery focusing on the role of imaging and clues to diagnosis.
Pulmonary edema
Postoperative pulmonary edema is a noncardiogenic form of edema primarily associated with increased capillary permeability and transient pulmonary microvascular hypertension. The cumulative incidence of postoperative pulmonary edema has been estimated at up to 7% in cohorts of patients undergoing surgery, with a relative mortality of approximately 11%, although with modern postoperative care the mortality rates are likely much lower.
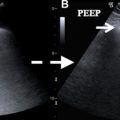
Anesthesia, volume overload from excessive intraoperative and postoperative fluid administration, and mechanical ventilation with high peak end expiratory pressure (PEEP) and tidal volumes can all contribute to microvascular injury. The acute development of negative intrathoracic pressure from laryngospasm after extubation also can increase total pulmonary vascular resistance, promoting pulmonary edema. Barotrauma, ischemia, and reperfusion damage produced by single lung ventilation with double-lumen endotracheal tubes during lung surgery are the most significant risk factors for postoperative edema. Extracorporeal cardiopulmonary bypass used during cardiac surgery is invariably associated with hemodilution and systemic inflammatory responses, which may trigger pulmonary edema. Pulmonary edema is reported to occur more commonly after right pneumonectomy because the left lung, which normally receives 45% of cardiac output, subsequently receives the entire cardiac output but lacks adequate lymphatic drainage to handle the volume.
Distinguishing cardiogenic from noncardiogenic edema following cardiothoracic surgery can be challenging because many patients who undergo such procedures usually have underlying cardiopulmonary comorbidities. Radiographic manifestations of pulmonary edema typically begin with peribronchial cuffing, Kerley B lines, and apparent thickening of the fissures from subpleural edema while more confluent lung opacity develops with more extensive edema, although overlap of findings is very common. Asymmetric distribution may occur following smaller resections and single lung ventilation or with prolonged lateral decubitus positioning ( Fig. 1 ). Widening of the vascular pedicle is an indirect sign of volume overload, reflecting distended mediastinal veins. Common computed tomography (CT) findings of edema include septal thickening and diffuse ground-glass opacities.

Acute lung injury
In the past, acute lung injury (ALI) was often used to describe either the radiological pattern or the clinical manifestations of patients with acute respiratory distress from immunologic-mediated injury to the lung. Particularly, ALI was used for patients with mild gas-exchange impairment (Pa o 2 /Fi o 2 ratio >200 mm Hg) who did not meet full criteria for acute respiratory distress syndrome (ARDS). The 2012 Berlin criteria revised the definition of ARDS and omitted ALI. ARDS now refers to rapidly progressive respiratory failure occurring within 7 days of an insult with Pa o 2 /Fi o 2 less than 300 mm Hg with minimum PEEP of 5 mm Hg and bilateral lung opacities consistent with edema present on radiographs or CT. Furthermore, the criteria state that the clinical presentation cannot be “fully explained by cardiac failure or fluid overload.” Although the Berlin criteria have improved the diagnosis of ARDS, interobserver agreement for some factors including radiographic findings is only moderate.
Pathologically, ARDS in its most severe form is characterized by diffuse alveolar damage, which is caused by a sudden alteration of the permeability of the alveolar-capillary membrane induced by an acute insult. Other histopathologic patterns that occur include organizing pneumonia and acute fibrinous and organizing pneumonia. Postoperative risk factors for ARDS include mechanical ventilation, lung infection, sepsis, transfusion-associated ALI, and aspiration. In one large series, patients undergoing pneumonectomy were more likely to develop ALI compared with those undergoing less extensive lung resection.
Patients presenting with clinical manifestations of ARDS often have radiographic findings, such as dependent atelectasis and patchy areas of lung consolidation, similar to those of infection and hemorrhage. The opacities become denser and more confluent over a period of just days with air bronchograms becoming apparent. CT findings include diffuse or patchy ground-glass opacities, which can have a geographic distribution with normal lobules located adjacent to abnormal lobules, and the subpleural regions of the lungs can be spared ( Fig. 2 ). For most patients, CT is not necessary, as it uncommonly provides additional useful information. Despite much improvement and recent success in therapy, ARDS is still associated with high in-hospital mortality rates of up to 40%.

Postoperative hemorrhage
All major surgical procedures include a risk of bleeding, and cardiothoracic surgical procedures are often associated with significant but expected intraoperative blood loss. Postoperative evidence of significant blood loss includes blood in a surgical drain, hemoptysis, tamponade, or hypovolemia. Most postoperative bleeding is self-limited, but severe or persistent hemorrhage may require surgical exploration and repair or endovascular embolization. Reactive bleeding develops within the first 24 hours after surgery and is usually related to vascular ligature slipping, dislodgment of an electrocautery clot, or, as in cardiovascular procedures and lung transplantation, dehiscence of a vascular anastomosis. Delayed postsurgical bleeding, with onset within the first 10 days, has numerous causes with surgical injury to vessels and inflammatory or infectious erosion being the 2 most important causes. Risk factors for postoperative hemorrhage include the type of surgery performed, vascular anatomic variants, and altered or weakened vessels from atherosclerosis, neoplasia, previous infection, chemotherapy, or radiation therapy. Compounding risk for hemorrhage are preexisting coagulopathy or concomitant antiplatelet or anticoagulation therapy. Postoperative chest radiography can detect the presence of subclinical bleeding or correctly localize the site of hemorrhage. CT can be used to confirm bleeding or to potentially identify sites of active bleeding.
Extrapleural Hematoma
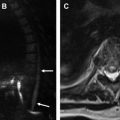
Clinically significant isolated extrapleural hematoma (EH) resulting from injury to an intercostal vessel is a rare consequence of rib resection and potentially can complicate thoracotomy or thoracoscopy. On chest radiographs, rapidly growing EH from arterial bleeding may appear as an expanding biconvex extrapulmonary opacity, whereas low-pressure venous injuries may be occult or present as small extrapleural collections ( Fig. 3 ). On chest CT and MR imaging, inward displacement of the extrapleural fat layer (extra-pleural fat sign) by a blood collection is characteristic. Contrast-enhanced imaging can show active bleeding in some cases.

Hemothorax
Postoperative hemothorax is reported to develop in fewer than 1% of patients after lung resection and up to 18% of lung transplant recipients. Pulmonary, bronchial, and intercostal vessels are all potential sites of bleeding. Patients may become hypotensive or have increased bloody output from a pleural drain. In the immediate postoperative period, chest radiography can be used to evaluate for developing pleural collections. Early onset, rapid expansion, and loculation suggest bleeding ( Fig. 4 ). However, radiographic detection of slow bleeding in the early postoperative period can be challenging, as the slow accumulation of pleural blood can be mistaken for the normal accumulation of serous fluid that follows pneumonectomy. CT typically shows a high-attenuation pleural collection and sometimes can reveal a site of active contrast extravasation.

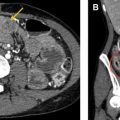
Mediastinal Hematoma
Mediastinal hematoma is another frequent presentation of postoperative bleeding and can be life threatening. Bleeding can result from manipulation of or injury to the pulmonary arteries and veins, azygos vein and its tributaries, and intercostal and internal thoracic arteries. Numerous radiographic findings suggest mediastinal bleeding, including increasing attenuation and increasing fullness of the mediastinum. Effacement of normal interfaces, such as the right paratracheal stripe and aortic arch along with displacement of the trachea and main bronchi, also suggest mediastinal hemorrhage ( Fig. 5 ).

Hemopericardium
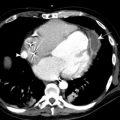
Hemopericardium can accompany mediastinal hematoma or occur in isolation. Slow accumulation of fluid, even large amounts (1000–1500 mL) can be tolerated well. However, rapidly developing small pericardial effusion (100–200 mL) can lead to tamponade. Rapid enlargement of the cardiac silhouette and progressive flattening of the left mediastinal and cardiac contours are highly suggestive radiographic findings of hemopericardium. On CT, high-attenuation blood products can be readily identified in the mediastinum, pericardium, or both ( Fig. 6 ). Contrast-enhanced CT sometimes can show the site(s) of active bleeding. Findings suggesting cardiac tamponade in addition to hemopericardium include dilation of the venae cavae and underfilling of the ventricles. The “flattened heart sign” is characterized by flattening of the anterior surface of the heart with decreased anteroposterior diameter. With severe tamponade, the cardiac chambers may have a concave morphology. Straightening of the right contour also can develop because of the relatively higher compliance of the right cardiac chambers. Bedside echocardiography provides rapid assessment of unstable patients.

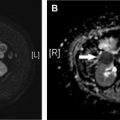
Diffuse Alveolar Hemorrhage
Diffuse alveolar hemorrhage, although extremely rare, may develop shortly after surgery, as a result of damage to the pulmonary microcirculation. Risk factors include preexisting pulmonary hypertension, mechanical ventilation, cytotoxic therapy, and inhaled halogenated anesthetics. Patients typically develop acute-onset dyspnea and hypoxia. Hemoptysis occurs in only approximately 60%. Hemoglobin levels can precipitously drop.
Chest radiographs typically show patchy or confluent bilateral lung opacities indistinguishable from edema or massive aspiration in most cases. CT findings are similar and include consolidation, ground-glass opacity, and centrilobular nodules. Superimposed septal thickening and intralobular lines are sometimes present, often more than 24 hours after the initial event. The abrupt onset of signs and symptoms and development of radiographic abnormalities is most of the clue to the diagnosis. Bronchoalveolar lavage is confirmatory.
Infectious Pneumonia
The incidence of postoperative infectious pneumonia after lung resection has been reported as high as 25% but is likely closer to 3% to 4% with routine use of preoperative antibiotics and aggressive postoperative pulmonary toilet. Prolonged atelectasis with bacterial colonization and aspiration are major contributors. Mortality rates have reported as high as 30%.
The diagnosis of postoperative pneumonia is often challenging, as the radiographic findings of lung consolidation and nodules may be delayed or obscured by underlying atelectasis and pleural effusion. Furthermore, fever and leukocytosis have a variety of potential sources in the postoperative period. CT can be helpful in selected cases in which the diagnosis is unclear because of its increased sensitivity for lung opacities as compared with portable chest radiographs. CT also can be helpful in cases in which there is little or no improvement despite appropriate therapy to assess for underlying abscess or empyema or to detect radiographic mimics of infection, such as venous infarct or lobar torsion.
Aspiration
Aspiration is a common complication among hospitalized patients, including those recovering from surgery. Aspiration can occur during anesthesia induction or with instrumentation, including endotracheal tube placement or removal or upper endoscopy. In addition, patients can aspirate from altered mentation, sedative effects from medication, or impaired aerodigestive function. Aspiration is of particular concern for patients undergoing esophagectomy because of altered anatomy and intraoperative injury to the recurrent laryngeal nerve, which can lead to impaired cough reflex. The injury to the airway and lungs depends on the volume and types of material aspirated. Aspiration can lead to acute respiratory failure, ARDS, and infection including empyema or abscess.
The typical radiographic findings of aspiration include gravitationally dependent, peribronchial consolidation, nodules, and bronchial wall thickening. Associated atelectasis can occur. CT imaging findings are similar to radiographs, but debris or secretions in the associated airways and nearby centrilobular nodules are readily apparent. Sometimes liquid or debris is retained in the esophagus.
Pleural complications
Empyema
Empyema is frank infection of the pleural space with an incidence after lung resection of 2% to 16% and occurring up to several weeks after resection, although rates have declined with improved surgical techniques and routine use of preoperative antibiotics. Early postoperative empyema typically results from residual infection in the pleural space, whereas later empyema often results from bronchopleural or esophagopleural fistulas.
Chest radiographic findings of postpneumonectomy empyema include expansion of the pneumonectomy space with straightening or convexity of the normally concave mediastinal margin of the pneumonectomy space, contralateral mediastinal shift, and thickening of the ipsilateral parietal pleura. Fluid levels also can develop in the pneumonectomy space, or the fluid level can drop as more gas enters. CT better delineates fluid loculations and underlying lung infection. Causative fistulas also can be apparent ( Fig. 7 ).

Management of empyema is with percutaneous drainage. Video-assisted thoracic surgery (VATS) drainage may be required for patients who fail more conservative therapy or in whom fistula treatment is also required, often with a muscle flap.
Chylothorax
Chylothorax refers to the persistent collection of lymphatic fluid within the pleural space. It usually results from obstruction or transection of the thoracic duct. Surgical procedures are the most common causes of traumatic thoracic duct injury. Chylothorax may occur following several cardiothoracic surgical procedures. Fewer than 1% of patients undergoing pneumonectomy develop chylothorax, whereas the incidence is up to 4% for patients undergoing esophagectomy. Chylothorax is usually indistinguishable from other causes of pleural effusion, and the diagnosis is confirmed by fluid analysis ( Fig. 8 ).

Airway complications
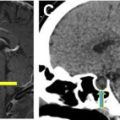
Tracheal Injuries
Intraoperative tracheal laceration is primarily related to airway management. Several factors contribute to tracheal injuries. Traumatic intubation and prolonged balloon cuff overinflation are mechanical factors, whereas underlying tracheal pathology can predispose patients to injury. Lacerations usually occur in the posterior membranous wall of the trachea due to lack of cartilage protection. A higher incidence of tracheal injury has been reported in women, thought to be related to a weaker membranous posterior membrane. The trachea also can be injured during esophageal surgery, such as esophagectomy. Tracheal laceration usually presents intraoperatively or in the immediate postoperative period with acute respiratory distress and hemoptysis.
Chest radiographs and CT typically show extensive pneumomediastinum, often with gas extending into the chest wall soft tissues. CT also can show mediastinal hematoma adjacent to the trachea as well as the defect in the tracheal wall ( Fig. 9 ).

Postoperative Persistent Air Leak
Postoperative air leaks are defined as a continuous air inflow into the pleural space because of an abnormal communication with the airways. An abnormal communication between the lung distal to a segmental bronchus is referred as an alveolar-pleural fistula (APF) and is the most common post-resection air leak. These typically result from direct injury to the visceral pleura and underlying lung during surgery. Incomplete or absent fissures together with diseased lung are major risk factors for APF in addition to impaired visibility in the operative field. APF is reported to be more common after upper lobe surgery (more on the right) or more extensive anatomic resection, such as lobectomy or bilobectomy.
Small postoperative air leaks are expected, with an incidence of 25% to 60%. However, in approximately 5% to 10% of patients, they may last more than 5 to 7 days and are considered persistent. In the normal postoperative course, small volume serosanguinous liquid accumulates in the pleural space with concurrent gas resorption, evident on serial chest radiographs and by decreasing pleural drain output. Stable or increasing gas in the post-resection space and failed reexpansion of the residual lung beyond the first postoperative week is suggestive of persistent air leak. Because most APFs are managed conservatively, chest CT is usually reserved for conservative treatment failures or when pleurodesis, endobronchial valve placement, or additional surgery is planned.
Bronchial Anastomotic Dehiscence
Bronchial anastomotic dehiscence can occur after sleeve lobectomy or lung transplant. The incidence after sleeve lobectomy has been reported to occur in up to 6% of patients. Extended lymph node dissection has been reported to increase the risk of bronchial anastomotic dehiscence because of excessive tension on the bronchial anastomosis site and subsequent bronchial wall ischemia. Bronchial anastomotic dehiscence is reported to occur in up to 10% of lung transplant recipients and develops between 1 and 4 weeks after transplantation. A variety of factors contribute to dehiscence and include surgical technique, donor-recipient size discrepancies, infection, and, most important, disruption of the bronchial arterial supply.
Radiographic findings suggesting dehiscence include new or worsening pneumomediastinum, pneumothorax, or both. CT is more sensitive and can show the defect or a collection of extraluminal gas adjacent to the anastomosis ( Fig. 10 ). Most bronchial anastomotic dehiscence is usually treated with an endobronchial stent. Surgical revision, often with muscle flap, is reserved for refractory cases.