Key Facts
- •
Osteoporosis is the most common metabolic bone disease and affects one in three women and one in five men over 50 years of age.
- •
Osteoporotic fractures are associated with considerable morbidity, mortality, and social and health-care costs.
- •
Effective therapies are available to reduce future fracture risk.
- •
Radiographic features (osteopenia, thinned cortex, reduced number, and thickness of trabeculae) may be present before fractures occur.
- •
Vertebral fractures are the most common osteoporotic fracture.
- •
Thirty percent to 50% of vertebral fractures may be present without symptoms.
- •
Vertebral fractures are powerful predictors of future fracture (vertebral five times; hip two times).
- •
The presence of vertebral fracture should be clearly and accurately reported by radiologists, and should stimulate further investigation/management.
- •
Other imaging techniques (magnetic resonance imaging [MRI], radionuclide imaging, and computed tomography) are helpful in identifying fractures that are occult on radiographs and in differentiating whether fractures are acute/chronic or due to other causes.
- •
MRI allows bone marrow edema present in reflex sympathetic dystrophy or migratory osteoporosis to be detected.
- •
Dual energy x-ray absorptiometry (hip, spine) is a quantitative method for confirming the diagnosis of osteoporosis.
Metabolic bone diseases affect bone as a tissue; all bones are involved histologically, but radiologic features are not always present. A variety of nutritional, biochemical, genetic, endocrine, and biochemical disorders can result in metabolic bone diseases.
Osteoporosis is the most common of the metabolic bone diseases and is now defined as “a systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase in bone fragility and susceptibility to fracture.”
Osteoporosis is the most common metabolic bone disease.
Not only is the amount of bone tissue reduced (quantitative abnormality of bone), but also the structural integrity and biomechanical strength of bone are compromised by reduction in number, thickness, and connectivity of trabeculae. Bones become brittle and fracture with little, or no, trauma (insufficiency [fragility] fractures), and these fractures are the cardinal clinical feature of osteoporosis. This is in contrast to rickets and osteomalacia in which there is a qualitative abnormality of bone (reduced mineral to osteoid ratio), the bones are soft and bend, and Looser zones may be present as a diagnostic radiologic feature (see Chapter 35 ).
Osteoporosis is characterized by a reduction in the number, thickness, and connectivity of trabeculae leading to reduced bone strength.
EPIDEMIOLOGY
Osteoporosis poses a significant public health problem, and in the Western world one in two women and one in five men over the age of 50 years will suffer a fracture in their lifetime. In the past 20 years there have been significant advances in knowledge of the epidemiology, pathophysiology, and treatment of osteoporosis. The fractures that occur in osteoporosis result in considerable morbidity and mortality for patients and incur large social and health care costs. The risk of fracture increases independently with advancing age and reduction in bone mass; approximately 70% of bone strength is related to bone mineral density (BMD).
Seventy percent of bone strength is related to bone density, and bone density can be measured by techniques such as dual energy x-ray absorptiometry (DXA) scanning.
The incidence of fracture varies with the population being studied; in Britain, 60,000 hip fractures, 50,000 wrist fractures, and 120,000 vertebral fractures occur per year, with associated health care costs of approximately £2 billion. In the United States, the age-adjusted incidence of insufficiency fractures in both men and women is 25% higher than in Britain and other areas of Europe, and the estimated cost of managing these fractures was $17 billion in 2001. The incidence of hip fracture has doubled over the past three decades, and it is predicted to continue to rise beyond what would be predicted from increased longevity in populations, particularly in the Far East.
Following hip or vertebral fracture, mortality at 5 years is about 20% greater than that expected, and mortality rate is highest in men aged over 75 years who have comorbidities. Most of the excess deaths occur in the first 6 months after hip fracture. One year after hip fracture, 40% of patients are unable to walk independently, 60% have difficulty with one essential activity of daily living, 80% are restricted in other living activities (e.g., driving, shopping), and 27% will be admitted to a nursing home for the first time.
Vertebral fractures are the most common type of osteoporotic fracture and may occur in the absence of, or after only minimal, trauma. In the United States, it is estimated that 25% of women aged over 50 years will have a vertebral fracture, and this will rise to 33% in those over 75 years. In Europe, there is a similar prevalence of 20% in women over 50, and a strong age dependency. In men, a prevalence of 20% over the age of 50 is found, but as more of these were present at an earlier age, traumatic events are presumed to be the etiology.
Vertebral fractures are the most common type of osteoporotic fracture. Hip fractures are the most life threatening.
BONE PHYSIOLOGY
Bones have an outer cortical (compact) shell and inner trabecular (net-like cancellous) bone tissue, which enable the skeleton to be light but strong. The relative amounts of each type of bone vary by skeletal site ( Table 31-1 ), and both contribute to bone strength. In research studies, quantitative measurements made in various anatomic sites and by different methods (DXA, quantitative computed tomography [QCT], and quantitative ultrasound [QUS]) may give complementary information about the amount and rates of change of the various bone components in diseases and with therapies.
| Anatomic Site | Cortical/Trabecular Ratio |
|---|---|
| Whole body | 80/20 |
| Hip | 60/40 |
| Lumbar spine | 50/50 |
| Distal radius | 95/5 |
| Ultra distal radius | 40/60 |
| Calcaneus | 5/95 |
Bone—which is composed of a matrix of collagen fibers, mucopolysaccharides, and inorganic crystalline mineral matrix (calcium hydroxyapatite)—is hard and strong and remains metabolically active throughout life (bone turnover), being continuously resorbed (by osteoclasts) and formed (by osteoblasts). This process can be modified by many factors, and bones consequently model and remodel throughout life from birth to maturity, maintaining their basic shape, repairing following fracture and responding to physical forces (i.e., mechanical loading). The overall strength of a bone is related to its hardness and other physical properties, size, shape, and architectural arrangement of the compact and trabecular bone.
Bone is a dynamic tissue, with bone resorption and bone production (bone turnover) occurring throughout life.
Bone formation (osteoblastic activity) and bone resorption (osteoclastic activity) constitute bone turnover, a process that takes place on bone surfaces and continues throughout life. Trabecular bone has a greater surface-to-volume ratio than compact bone, is about eight times more metabolically active, and therefore has a larger turnover than cortical bone. In adult life and under normal circumstances, bone formation and resorption are linked in a consistent sequence; precursor cells are activated to form osteoclasts, which erode a fairly constant amount of bone. After a period of time (about 3 to 4 months), the bone resorption stops and osteoblasts are recruited to fill the eroded space with new bone tissue. Under normal circumstances, this osteoblastic and osteoclastic activity is coupled and constitutes the basal multicellular unit (BMU). There will be numerous BMUs throughout the skeleton at different stages of this cycle, and the amount of bone in the skeleton at any moment in time depends on peak bone mass attained during puberty and adolescence and the balance between bone resorption and formation. If there is uncoupling of this process, with either excessive osteoclastic resorption or defective osteoblastic function, then there is a net loss of bone (osteoporosis). Increased activation frequency of resorption units results in high bone turnover states (hyperparathyroidism, postmenopausal bone loss, Paget’s disease). Therapy with bisphosphonates reduces the activation of resorption units by inhibiting osteoclastic function, and the consequent reversal of the mineral deficit contributes to the increase in BMD that occurs with such therapy.
Normally, bone turnover is coupled so that bone production and destruction are balanced; uncoupling results from excessive resorption or defective bone production.
The bones grow during the first two decades of life with a pubertal spurt during adolescence. Skeletal maturity is achieved at an earlier age in girls (16 to 18 years) than in boys (18 to 20 years). Following attainment of skeletal maturity, a period of consolidation follows during which peak bone mass is achieved. For cortical bone, this is reached at about 35 years of age and a little earlier for trabecular bone. Although the long bones grow in length at the metaphyses, they are remodeled in shape during development by endosteal resorption and periosteal apposition. The size and shape of the skeleton and its individual bones are determined by genetic factors, but they are influenced by endocrine and local growth factors, nutrition, and physical activity. Remodeling allows the skeleton to adjust to mechanical forces to which it is exposed. There is considerable variation in skeletal size and weight both within and between race/ethnic groups. Dark-skinned people tend to have larger and heavier bones than whites, and some Asian groups tend toward a small skeletal mass and size. Although genetic factors are important, they are modified by environmental differences such as diet and physical activity.
The amount of bone in the skeleton at any moment in time depends on peak bone mass and the balance between bone resorption and formation. Osteoporosis is not a single disease entity but an end result of many disease processes. It may result from defective skeletal accretion during bone growth and development in childhood and adolescence or defective osteoblastic function (e.g., in glucocorticoid therapy). Alternatively, it can result from disease processes in which bone resorption exceeds new bone formation, resulting in a net loss of bone mass and consequent compromise in skeletal strength.
Postmenopausal bone loss is usually due to excessive bone resorption.
Bone can also be lost focally in specific anatomic regions, such as occurs in periarticular regions in the inflammatory arthritides (e.g., metacarpal and proximal interphalangeal joints in rheumatoid arthritis). This loss of bone is related to hyperemia and the release of cytokines, which stimulate local osteoclastic bone resorption. This is perhaps more appropriately described as “osteopenia” than osteoporosis.
In the past, treatment of established osteoporosis was limited and unsatisfactory and consisted mainly of hormone replacement therapy [HRT] for postmenopausal osteoporosis. However, in recent years the introduction of bone protective therapies (bisphosphonates, selective estrogen receptor modulators [SERMs], strontium ranelate) and the anabolic agent teriparatide (parathyroid hormone) have been shown to result in modest increases in bone mineral density (incremental changes of 6% to 12%) and, more importantly, reduce future fractures to a much greater extent (reductions of 40% to 70%). Treatment strategies have generally favored prevention of osteoporosis by maximizing peak bone mass, minimizing age-related and postmenopausal bone loss (HRT in premature menopause), and avoidance of risk factors (e.g., smoking, excessive alcohol) with adequate dietary intake of calcium and vitamin D (for supplementation, this is achieved by 1 g of elemental calcium and 800 units vitamin D) and regular weight-bearing physical activity. Bisphosphonates are increasingly being used to treat osteoporosis due to a variety of causes in children as well.
Compliance and persistence with oral bone-protective therapies taken daily is relatively poor, being approximately 50% at 1 year. This situation has been improved with the introduction of monthly dosing with certain bisphosphonates and intravenous administration of others. It is with the latter, rather than with oral preparations, which are being given for hypercalcemia in patients with metastatic cancer, that reports have emerged of the uncommon complication of osteonecrosis of the jaw.
Adequate analgesia should be given to manage pain during an acute fracture episode or appropriately as improves the quality of life thereafter for those severely affected by osteoporosis; physiotherapy may also be helpful.
CLASSIFICATION AND CAUSES OF OSTEOPOROSIS
Osteoporosis can be generalized, involving the whole skeleton, or regional, in which only a segment, or focal area, of the skeleton is affected. There are many etiologies of both types of osteoporosis.
Generalized Osteoporosis
This may be primary ( Box 31-1 ) in origin or secondary to other disorders ( Table 31-2 ), which either reduce acquisition of peak adult bone mass or increase age-related bone loss. Osteoporosis can occur in adults and children ( Box 31-2 ).
- •
Idiopathic juvenile
- •
Osteoporosis of young adults
- •
Osteogenesis imperfecta
- •
Postmenopausal (estrogen decline)
- •
Senile (age related)
| Endocrine | Nutritional |
|---|---|
|
|
| Hereditary | Hematologic |
|
|
| Other | Other |
|
|
PRIMARY OSTEOPOROSIS
- •
Osteogenesis imperfecta
- •
Idiopathic juvenile osteoporosis
- •
Osteoporosis of young adults
SECONDARY OSTEOPOROSIS
- •
Systemic long-term glucocorticoid therapy
- •
Hypogonadism, primary or secondary
- •
Prolonged immobilization (e.g., cerebral palsy, paraplegia)
- •
Chronic inflammatory disease (e.g., juvenile inflammatory arthritis)
- •
Chronic liver and inflammatory bowel disease
- •
Neoplasia and therapy (e.g., leukemia)
- •
Other, including cystic fibrosis, anorexia nervosa
Primary Osteoporosis
Idiopathic juvenile osteoporosis (IJO) is a rare, self-limiting disease in prepubertal children aged 8 to 14 years who have previously been healthy. For a period of about 2 to 4 years, growth arrest and fractures occur, with loss of both cortical and trabecular bone and a wide spectrum of severity. Only one or two vertebral fractures are present in mild disease, but in more severe cases fractures involve all vertebrae and the extremities, particularly the metaphyseal region of the distal tibia. In a few patients, these can result in severe kyphoscoliosis, deformities of the extremities, and even death from respiratory failure due to deformity of the thorax. The disease is reversible and remits spontaneously, with the residue of only a mild or moderate kyphosis, short stature, and some bone deformity following fractures. Investigations indicate uncoupling of bone turnover with increased resorption and decreased formation. The condition must be differentiated from osteogenesis imperfecta (no blue sclerae) and other forms of juvenile osteoporosis. Other important differential diagnoses in children with vertebral fractures are hypercortisolism (e.g., Cushing’s disease or systemic glucocorticoid therapy) and leukemia ( Box 31-2 ) ( Figure 31-1 ).




Osteoporosis of young adults is a heterogeneous condition that occurs in young men and women equally, usually runs a mild course with multiple vertebral fractures occurring over a decade or more, and is associated with height loss. Fractures of metatarsals and ribs are also common, and hip fractures may occur. The cause of the condition is obscure, and it may simply be that inadequate bone mass has been accrued during skeletal growth; some affected individuals may have a mild variant of osteogenesis imperfecta. Exceptionally, osteoporosis may present during pregnancy, but whether this is a causal or coincidental association is unknown.
Osteogenesis imperfecta (OI) or “brittle bone” syndrome, results from mutations affecting either the COL1A1 or COL1A2 genes of type I collagen. A number of inherited disorders of connective tissue can result in osteoporosis ( Table 31-2 ). Although the disease is usually apparent at birth or in childhood, more mild forms of the disease may not become apparent until adulthood, when affected individuals present with insufficiency fractures and osteopenia. A common classification (types I to IV) of OI is one devised by Sillence. (See Chapter 32 .) The important characteristics in this classification include blue sclerae, the severity of the disorder, and the mode of inheritance (dominant, recessive, sporadic/new mutation), although accurate classification is difficult because of phenotypic overlap. Subjects who do not have dental involvement are designated as group “A,” and those with dentinogenesis imperfecta are designated as group “B.”
Type I is the mildest and most prevalent form of OI and may only become apparent in adulthood. There is a history of fractures, generally dating back to childhood. In children the fractures may become radiographically and clinically apparent as the child becomes more active (5 years and older) and may be overt fractures of long bones and vertebrae or microfractures of the metaphyses. If present in infancy, these features may resemble those found in nonaccidental injury. The differential diagnosis can usually be resolved by the presence of associated extraskeletal manifestations of OI (blue sclera, dentinogenesis imperfecta) or a family history of OI, so bone biopsy is seldom required for diagnosis. Stature is short, with only 10% of patients being of normal height; there is joint laxity, blue sclerae, and pre-senile hearing loss. Transmission is by autosomal dominant trait, and radiologically the bones are usually reduced in density, although some patients may have normal bone density. Bones may be narrow and undertubulated (gracile) or normally modeled. Vertebral fractures occur in the fourth decade, and scoliosis, if present, is mild.
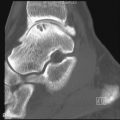
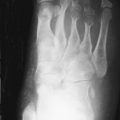
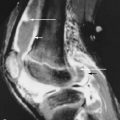
Type II (lethal perinatal) OI results in affected infants being small, with deep blue sclerae and short and deformed limbs due to multiple fractures ( Figure 31-1 ). Fractures involve ribs, and death is usually the result of pulmonary insufficiency making survival beyond the first 3 months of life rare. Other complications include brain and spinal cord injury. Radiologically, multiple fractures are present with a characteristic “concertina” deformity of the lower limbs; the ribs may appear “beaded” due to multiple rib fractures ( Figure 31-1 ), which can occur in utero. The cranial vault is severely undermineralized and may be distorted by molding. Wormian (intrasutural) bones in the occipital and parietal region ( Figure 31-1 ) and platyspondyly are noted.
Type III (severe progressive) OI is inherited as an autosomal recessive trait, with fractures usually present at birth and involving long bones, clavicles, ribs, and cranium with resulting deformity. Although size at birth is normal, retardation of growth is evident in the first year of life, and many patients reach only 3 to 4 feet in height in adulthood. As growth proceeds, increasing deformity of the calvaria occurs, with resulting facial distortion, malocclusion, and mild prognathism, basilar invagination, and progressive hearing loss. Sclerae are blue at birth, but this diminishes with age, and sclerae are white in adults. Vertebral fractures occur at an early age (see Figure 31-1, C ) and contribute to the progressive and severe kyphoscoliosis that develops during childhood. Patients tend to be wheelchair-bound because of the progressive deformities resulting from fractures. Complications include progressive pulmonary insufficiency through distortion of the thorax. Radiologically, the bones may be slender or broad due to recurrent fractures, and epiphyses are abnormal, with expansion and islands of calcified (“popcorn”) cartilage. As in other forms of OI, the incidence of fracture declines following puberty.
Type IV (moderately severe) OI is inherited as an autosomal dominant trait and can vary in severity and be confused with either type I or type III OI. There is generally more severe osteopenia and extensive bone deformity than in type I. The sclerae are blue in children, which may persist into adulthood but may also fade to white. Patients are short in stature with abnormal molding of the calvarium and basilar invagination in a high proportion of patients. Bones of the spine and limbs are osteoporotic and dysplastic, resulting in scoliosis and deformity, particularly of the pelvis. Joint laxity can result in dislocation, particularly of the ankle or knee.
Although there is no cure for OI, treatments have been aimed at increasing bone strength to prevent fracture and maintain physical activity. Bisphosphonates, either oral (alendronate) or intravenous (pamidronate, zolendronate), have been demonstrated to increase bone mass, restore vertebrae to their more normal shape, and reduce fractures in small study groups ( Figure 31-2 ). If cyclical doses of bisphosphonates are given (e.g., intravenous pamidronate, which is given every 3 months), then sclerotic lines result in the metaphyses of long bones in children (see Figure 31-2 ).



Postmenopausal (Type I) Osteoporosis
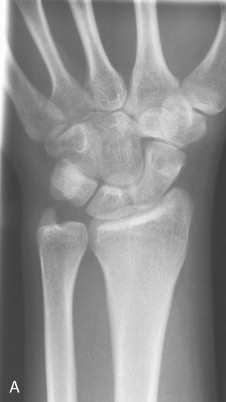
At menopause, estrogen levels fall, and as a consequence all women lose bone at this time, some losing trabecular bone at a rate three times greater than normal (2% to 10% per annum). The bone loss is greatest during the first 4 years after menopause. The condition characteristically becomes clinically evident in women 15 to 20 years after the menopause. Fractures occur in sites of the skeleton rich in trabecular bone, including the vertebrae and distal forearm (Colles fracture) ( Figure 31-3 ). In premature menopause, bone loss can be prevented by HRT up to the age of natural menopause (approximately 50 years of age). However, it is now believed that HRT should not be used long term after this age for the purpose of bone protective therapy as there are other more specific and effective bone agents now available and because of the increased risk of breast cancer and cardiovascular events with long-term use of HRT in elderly women.

HRT should not be used long term after menopause as bone protective therapy as there are other more specific and effective bone agents available and there is increased risk of breast cancer and cardiovascular events.
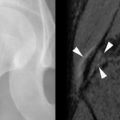
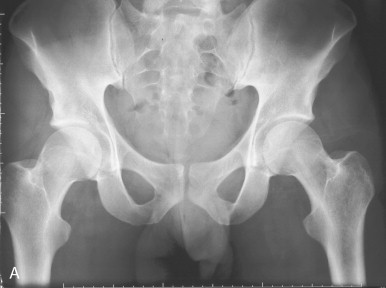
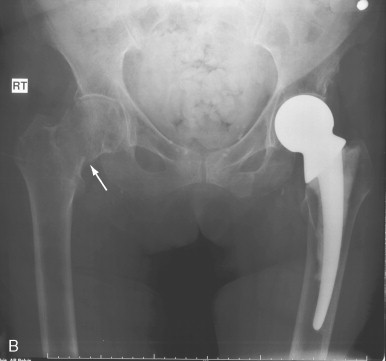
Senile (type II) osteoporosis occurs in both men and women of 75 years or older and is due to age-related bone loss related to impaired bone formation and secondary hyperparathyroidism that occur with advancing age. The latter is a consequence of reduced calcium absorption from the intestine secondary to decreased production of the active metabolite of vitamin D (1,25[OH] 2 D) in the kidneys of the elderly. There is reduction in both cortical and trabecular bone, and the syndrome manifests mainly as hip fractures ( Figure 31-4 ) and wedge fractures of the vertebrae. However, fractures may also occur in the proximal tibia, proximal humerus, and pelvis.
Secondary Osteoporosis
Many conditions can result in osteoporosis and may be of endocrine, nutritional, hereditary, hematologic, or other origin ( Box 31-2 ). Radiologically, the appearances may be indistinguishable from involutional (senile) osteoporosis, although some diseases may have specific and diagnostic radiologic features (e.g., subperiosteal erosions of the phalanges in primary hyperparathyroidism). In glucocorticoid excess (endogenous and exogenous), there is reduced bone formation due to a direct effect on the osteoblasts, with increased osteoclastic activity, probably mediated through secondary hyperparathyroidism stimulated by reduced gastrointestinal absorption of calcium. There is also evidence that glucocorticoids induce premature apoptosis of both osteoblasts and osteoclasts. The adverse effect is primarily on trabecular bone, and fractures occur particularly in the vertebrae and ribs; the latter may heal with profuse callus formation.
Myeloma may produce generalized osteoporosis.
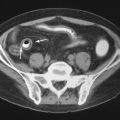
Thyrotoxicosis stimulates catabolic activity and increases osteoclastic bone resorption and may be severe enough to occasionally cause recognizable osteoporosis. Long-term heparin therapy has also been reported to cause osteoporosis, possibly due to release of collagenase from lysosomes. Formation of osseous matrix also requires other substances such as protein and vitamin C (for the formation of collagen). Hence osteoporosis may develop in deficiency states involving lack of proteins (e.g., in starvation or severe malnutrition) or in scurvy. Hematologic disorders can result in osteoporosis; in hemolytic anemias, there is marrow hyperplasia and expansion, which causes change in bone modeling, thinned bone cortex, and a coarse and prominent net-like trabecular pattern ( Figure 31-5 ). Myeloma can cause generalized osteoporosis, but there may also be “punched-out” lytic lesions in bones (see Figure 31-5 ), particularly in the skull. Finally, a number of other disorders including hepatic disease and chronic alcoholism may cause osteoporosis by various mechanisms, many of which are still not clearly understood at the present time.


REGIONAL OSTEOPOROSIS (OSTEOPENIA)
In some conditions, bone loss may not be generalized but localized to a specific anatomic region ( Box 31-3 ).
- •
Disuse (e.g., stroke, poliomyelitis, cerebral palsy, spinal cord injury)
- •
Reflex sympathetic dystrophy (Sudeck’s atrophy)
- •
Regional migratory osteoporosis
- •
Periarticular osteoporosis (e.g., inflammatory arthritis)
Disuse osteoporosis occurs through inactivity and failure to load the skeleton normally ( Figure 31-6 ). The growth and development of the skeleton and maintenance of bone health throughout life depends on normal activity and loading of the skeleton. If, for any reason, there is inactivity (e.g., stroke, polio, spinal cord injury), there will be localized osteopenia involving the part of the skeleton affected. Such a process may be acute (e.g., following a fracture) or chronic (e.g., following a stroke, cerebral palsy) ( Figure 31-6 ).