- •
Chest pain is one of the most common ED chief complaints and represents approximately 5% of all ED visits.
- •
Although triage of low-risk and high-risk patients who present to the ED is straightforward, many patients fall in the clinically intermediate risk category and comprise a unique decision-making challenge that often results in either unnecessary expensive testing or inappropriate early discharging.
- •
The ischemic cascade dictates that the mismatch between myocardial oxygen supply and demand during an ischemic event first results in regional flow heterogeneity, followed by development of perfusion defects, then mechanical regional wall motion abnormalities, and finally the development of electrical defects and clinical symptoms.
- •
The role of radionuclide MPI to triage patients was robustly studied in large-scale randomized controlled trials and was demonstrated to be a safe and effective strategy to triage patients compared with routine evaluation.
- •
CCTA has evolved into a standard diagnostic tool backed by evidence from several multicenter randomized trials that consistently show favorable impact on decision making in the ED.
- •
Despite the favorable data in small single-center trials of CMR in the decision making for chest pain in the ED setting, its effectiveness has yet to be evaluated in a large-scale multicenter trial. Availability of testing for this type of patient may limit its use.
- •
Bedside echocardiography is a helpful diagnostic tool that improves the sensitivity compared with routine clinical testing to detect infarct but must be performed in the presence of ongoing symptoms to be useful for detecting ischemic wall motion abnormalities.
- •
Future studies will likely focus on those patients with “border zone” elevation of high-sensitivity troponins, who are not clearly stratified into very low or very high ACS risk by the initial and early follow-up troponin values.
Scope of the problem
Chest pain is one of the most common chief complaints in subjects presenting to the emergency department (ED), representing 5% of all ED visits. , In the United States, approximately seven to eight million individuals present to the ED each year for evaluation of chest pain, at an annual cost of approximately five billion U.S. dollars for the care they are provided. Despite this high frequency, only 10% to 20% of these visits are truly the result of an underlying cardiovascular cause and, among those, only 5% are caused by a life-threatening acute coronary syndrome (ACS).
Therefore the majority of patients who present to the ED with chest pain are at low risk for developing adverse cardiac events in the short term and may therefore be safely discharged from the ED without the need for hospital admission once they are identified as being at very low risk. Identifying such patients quickly, however, has been a challenge, and the pursuit to accurately quantify risk among ED patients with chest pain has been an area of robust investigation for decades.
Because the goal of evaluation in the ED is to triage patients in a rapid and safe manner, various clinical prediction models were developed to risk-stratify such patients, aid in cutting costs, and reduce overcrowding in the ED. These models were often based on specific patient-related clinical features and objective data collected in the ED and were an initial attempt to quantify the likelihood of ACS to aid in triage decisions, rather than have clinicians rely on their own intuition and experience. Although the models stratified patients more accurately than clinicians, their performance was not sufficient to engender adoption because they were likely to miss cases of ACS events. Although the risk of missing ACS among ED patients is overall low and reportedly in the range of 1% to 2%, failure to accurately recognize these patients is not without consequences and is at the expense of up to a twofold increase in the risk for mortality compared with patients who are admitted and evaluated in the hospital. , , Further more, although this 1% or so epidemiologic risk has been considered acceptable among experts, individual cases of missed diagnoses have led to important legal concerns and malpractice litigation among ED clinicians. These safety issues have understandably resulted in the adoption of a very conservative approach to triage in such patients, including a common practice of holding patients for observation, ordering costly diagnostic tests, and maintaining a very low threshold for hospital admission.
The lack of sufficient and rapid stratification encouraged clinicians and investigators to develop strategies to further improve the risk stratification process and reduce the risk for missed ACS events. Of particular interest and focus has been the goal of rapidly identifying very low-risk patients (commonly quantified as being at <1% risk of ACS or myocardial infarction [MI] over the subsequent 30 days from presentation), with the implication that such patients could be safely discharged directly from the ED. Over the past 20 years, substantial evidence from randomized strategy trials has evolved.
Evaluation of patients with chest pain in the emergency department
The aim of evaluating patients with chest pain in the ED is to identify ACS early on and rapidly impose an aggressive therapeutic plan. History-taking and a 12-lead electrocardiogram (ECG) remain the most important initial diagnostic tools that are typically readily available for all subjects coming to an ED with chest pain. Because initial ECG changes detect less than two-thirds of patients with ACS, ECGs are often serially tested at 20 to 30 minute intervals for patients with persistent symptoms. , In clinical practice, however, only a minority of patients have typical anginal symptoms with a clearly interpretable ECG demonstrating new or dynamic changes (clear-cut ST-segment elevation or depression) consistent with ischemia. The majority of patients either present with atypical symptoms or have ECGs that are difficult to interpret because of either baseline changes, findings of unknown chronicity, or complete absence of ECG features of ischemia. Thus, any approach relying on history and ECG will, by its nature, be limited and insensitive. The main importance of the initial ECG is to quickly capture the 10% to 15% of presenting subjects who will have obvious ST-segment changes of elevation or depression to rapidly move them into an ACS treatment pathway.
The development of cardiac troponin assays, and more recently high-sensitivity troponin assays, has changed the standard by which patients with chest pain are evaluated in the ED. Troponin has now become the fundamental cornerstone in the diagnosis of MI and has been integrated into the iterations of the universal definition of MI, including the most recent iteration. Although prior non–cardiac-specific enzymes, namely aspartate transaminase, lactate dehydrogenase, creatine kinase, and myoglobin, were available before troponin, they were not diagnostically helpful for triage decisions in the ED because they lacked specificity to cardiac myocytes and their detection times in patient sera after the onset of an ACS event were markedly delayed.
Serial assays of high-sensitivity troponin at presentation and 1 hour later have been associated with high negative predictive value of 99% to rule out MI, whereby the absolute change of the level within 1 hour may be used as a surrogate for absolute change over 3 to 6 hours (specific cutoff levels are assay-specific). Thus the European Society of Cardiology (ESC) guidelines have adopted the 0/1-hour algorithm to rapidly diagnose and safely and effectively triage patients with suspected ACS/ non-ST-elevation myocardial infarction (NSTEMI). When further validated among 4368 patients presenting to the ED, the 0/1-hour algorithm proved to be safe for triaging patients, particularly for ruling out NSTEMI among those with negative serial assays or ruling in NSTEMI among those with elevated initial assay or a significant change in troponin concentration over 1 hour. Therefore, based on the available risk stratification models, it seems the combination of patient clinical features, ECG, and serial troponin is necessary for adequate risk stratification, with a negative predictive value effectively approaching 100%. These important studies focus on one end of the spectrum of patients, those with negative high-sensitivity troponin values within a relatively short time frame from ED presentation, and identify a group of patients for whom safe direct discharge from the ED is reasonable.
Despite several advantages over earlier generation assays, the high-sensitivity nature of contemporary troponin assays also brings with it a new set of limitations. In patients presenting with new-onset chest pain, high-sensitivity troponin has been demonstrated to be elevated in many non-ACS cardiac conditions, including arrhythmias, acute decompensated heart failure, myocarditis, and noncardiac conditions such as sepsis and liver disease.
Perhaps, most importantly, real-world registries have demonstrated that approximately 30% of patients have “border zone” values of high-sensitivity troponin that do not fall in either category to conclusively rule in or rule out acute cardiac ischemia. Between the two extremes of risk lies an intermediate group that does not fulfill the criteria for either. For example, in one large registry among patients with a “rule-out” range of high-sensitivity troponin I or T, the prevalence of NSTEMI was 0.3% and 0.2%, respectively, well below the 1% risk threshold to potentially enable direct discharge. Among those with “rule-in” ranges, prevalence of ASC/NSTEMI was very high. Nevertheless, among those patients with borderline values of high-sensitivity troponin I or T in the 0/1-hour testing algorithm, NSTEMI was ultimately diagnosed in 7.2% and 14.2%, respectively. Thus, even though 80% to 90% of such patients will not ultimately rule in for an ACS, these patients have an intermediate likelihood of ACS at that early time of presentation and need further evaluation.
In clinical practice, these patients comprise a diagnostic challenge to clinicians because the appropriateness for admission and further testing remains unclear and the safety of discharging directly from the ED is also not practical because most clinicians will not be comfortable with the fact that an ACS has been adequately ruled out. Accordingly, additional risk stratification in this intermediate risk group is an unmet need. With the application of cardiac imaging modalities in this setting, the utility of these diagnostic tests as a means of risk stratification may address the uncertainty surrounding these intermediate-risk patients who present to the ED with symptoms suggestive of ischemia but with nondiagnostic ECG findings and high-sensitivity troponin findings that do not conclusively place the patient at either extreme of the risk spectrum ( Fig. 11.1 ). In contemporary practice, with the increasing deployment of high-sensitivity troponin assays, it is this intermediate or border zone group of patients who are most likely to benefit from the information provided by imaging.

The ischemic cascade and the rationale for use of noninvasive imaging
The rationale to use noninvasive imaging to interrogate various underlying pathophysiologic factors in the evaluation of chest pain in the ED is based on the sequence of events that occur in the ischemic cascade . During an ischemic event, a series of occurrences ensue in a hierarchical and sequential manner. Early on, the initial mismatch between myocardial oxygen supply and demand during an ischemic event is associated with potentially detectable regional coronary flow heterogeneity between subendocardial and subepicardial perfusion and/or between one myocardial region and others. This mismatch leads to the development of perfusion defects that are detectable on imaging. Interestingly, these defects may persist for several hours after the inciting event, even after the resolution of clinical symptoms. With worsening of the supply/demand mismatch, metabolic changes begin to occur that initially cause diastolic functional abnormalities and subsequently systolic dysfunction manifested by regional systolic wall motion abnormalities. Finally, electrical abnormalities, manifested as ischemic findings on ECG, and then clinical manifestations, such as chest pain, are the later events in the ischemic cascade to develop. ,
Therefore patients who present to the ED with ongoing chest pain due to myocardial ischemia are likely to already have perfusion defects and wall motion abnormalities on arrival, potentially detectable by noninvasive imaging. The usefulness of imaging to rule out an ACS is based on the sensitivity of the test (in turn based on its spatial and temporal resolution to pick up subtle changes) as well as the timing of imaging. Many patients are not continuously symptomatic during an ED evaluation, and the resolution of symptoms may be associated with a resolution of the abnormality to be detected by imaging. This appears to be particularly important for detecting wall motion abnormalities because they resolve more quickly than perfusion abnormalities.
Therefore the use of noninvasive imaging in the ED setting among patients with symptoms suggestive of ischemia confers a significant advantage in the detection of ACS and may be a useful tool for diagnosing and triaging these patients and in the decision-making process.
Patient-centered applications of noninvasive imaging in patients with suspected acute cardiac ischemia
Case vignette 1: Radionuclide myocardial perfusion imaging
A 65-year-old female with hypertension, type 2 diabetes mellitus on insulin therapy, and hyperlipidemia presents to the hospital with an episode of chest pain, with several features that are atypical for angina secondary to myocardial ischemia. The presenting ECG shows normal sinus rhythm with no evidence of ischemia. Initial laboratory work-up, including high-sensitivity troponin I assay at arrival and also 3 hours from presentation, does not conclusively rule in or rule out ACS. The ED physician is not completely comfortable discharging the patient based on the risk factor profile and needs additional information to inform the triage decision. Rest single photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) is ordered. SPECT images performed around 45 minutes after tracer injection at rest show a normal resting perfusion pattern ( Fig. 11.2 A). Given the normal MPI results and the data supporting strong negative predictive value, the patient is discharged from the ED and scheduled for a follow-up stress test later in the week.

Radionuclide MPI uses either SPECT or positron emission tomography (PET) to evaluate myocardial perfusion. To this end, radionuclide MPI uses a radiotracer to determine the distribution of blood flow within the myocardium (see Fig. 11.2 A). In the 1970s, it was demonstrated that an abnormal region of 201 thallium uptake after an injection of the tracer at rest in the ED was present when imaged early after injection in 100% of patients with acute MI within 6 hours of symptom onset and was therefore a radiotracer of interest in the evaluation of patients with chest pain. Nevertheless, 201 thallium has the property of redistribution out of areas of high initial uptake more quickly than from areas of low initial uptake within a few hours after administration, minimizing contrast between regions and undermining the ability to see defects that would have been present if imaged earlier. The need to image quickly after tracer injection was a major factor limiting its clinical use in this setting, but the concept of rest imaging to assess patients with suspected ACS was established.
Alternatively, 99m technetium ( 99m Tc) tracers such as sestamibi or tetrofosmin are characterized by minimal redistribution over time, a relatively short half-life, predominant photon emission of higher energy (140 keV), and lower likelihood for attenuation artifact. These appealing properties have allowed 99m Tc agents to be the preferred radiotracer agents, essentially overcoming the limitations associated with 201 thallium because image acquisition could be performed even 1 to 2 hours after injection and the perfusion pattern would reflect perfusion at the time of tracer injection (see Fig. 11.2 B). This enabled the technique to be more practically applied because injection could be done in the ED, but then the patient could be transferred for imaging to a separate imaging suite.
The clinical use of SPECT MPI for assessing ED patients with suspected ACS using 99m Tc was first described in 1991 among patients who were already hospitalized with suspected ACS. The sensitivity and specificity of the test were 96% and 79%, respectively, among patients with active symptoms for the detection of angiographic CAD, whereas the use of ECG at the time of injection had a sensitivity of only 35% despite the presence of symptoms. In the ED setting, the sensitivity and specificity of 99m Tc SPECT MPI have been consistently high across studies, as has the negative predictive value.
When further evaluated in patients with suspected ACS, SPECT imaging was demonstrated to provide not only important diagnostic information but also prognostic data and was useful to risk-stratify patients who presented to the ED with chest pain. In an initial report with a small number of patients, when patients who had undergone 99m Tc-labeled sestamibi SPECT were followed up through 18 months, none of the patients with normal resting perfusion had major cardiac events (n = 34), whereas those with a positive defect (n = 30) had an event rate as high as 20% during the follow-up period. In a subsequent larger study, when patients in the ED with nondiagnostic ECG underwent 99m Tc-labeled sestamibi SPECT, 8% of patients with an abnormal scintigram had events compared with no events in 87 patients with a normal scintigram when followed through 90 days. In the intermediate to long-term, the annual incidence of nonfatal MI or cardiac death has been as low as 0.5% to 0.8% among patients with normal findings on SPECT versus 7% among those with abnormal findings. Notably, this association between abnormal SPECT imaging and the risk for late cardiac events and death is independent of other clinical risk factors, such as ECG findings of ischemia, history of congestive heart failure, history of MI, or history of diabetes mellitus. Based on these data, it is compelling that the addition of SPECT imaging to clinical data helps improve the risk stratification process for patients with ischemic symptoms in the ED. Thus the diagnostic and risk stratification data for rest MPI in the setting of suspected ACS in the ED all suggested that subjects with normal resting images had a very low likelihood of ACS and also a low risk for adverse outcome events over short-term or medium-term follow-up. The observational data supported the “efficacy” of MPI in this setting; that is, the test was performing well for its stated purpose, with the data generally emanating from centers expert in its use. Whether those data translated into strong “effectiveness” (i.e., did the test perform well in more real-life situations to favorably affect decision making) had not at that point been demonstrated. To do so requires randomized trials comparing some relevant outcome after the use of the test versus not using the test.
The effectiveness of MPI testing in triaging ED patients was evaluated prospectively in two randomized controlled trials. In 2000, Stowers et al. enrolled a small number of intermediate-risk patients with chest pain who had no evidence of ischemia on ECG (n = 46 patients). All patients had 99m Tc-tetrofosmin SPECT imaging at rest and were then randomized to either perfusion imaging–guided strategy or a “conventional” strategy with the perfusion imaging data blinded to the caregivers. Patients randomized to the perfusion imaging–guided strategy had shorter length-of-stay and lower in-hospital costs, but there was no difference in the event rates in-hospital and through 30 days of follow-up between the two groups. Notably, per this trial’s protocol design, the clinicians in the ED were offered protocolized guidance with regard to management and triage after receiving information obtained from the SPECT imaging, so the decision-making process was not predominantly based on the discretion of the clinician in the ED. Thus this, trial did not truly assess ED clinicians’ independent incorporation of the test results into their decision-making process.
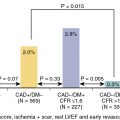
The ERASE Chest Pain (Emergency Room Assessment of Sestamibi for Evaluation of Chest Pain) trial was a prospective, large-scale randomized trial that more rigorously evaluated the effectiveness of resting MPI on the triage decision by ED physicians (n = 2475 patients). Symptomatic patients with chest pain and nondiagnostic ECGs were randomized to either the usual ED evaluation strategy versus the usual strategy plus incorporation of the results of rest 99m Tc-sestamibi SPECT imaging. The overall decision making and triage in the ERASE Chest Pain trial was entirely at the discretion of the clinician, which was a notable difference from the study by Stowers et al., and thus, this was truly an effectiveness trial, one in which the effects of the imaging data are assessed for their impact in practice. Among patients with chest pain whose final diagnosis was acute cardiac ischemia, there was no overall difference between the two strategy groups in triage decision making, and there were no differences in outcomes between the usual care group versus the imaging group. Among those whose final diagnosis was not ACS, however, and therefore the “correct” triage would have been ED discharge to home, the group randomized to the SPECT imaging strategy was shown to have a reduction in unnecessary admissions. In these patients, the occurrence of hospitalization was 52% among those randomized to the usual evaluation strategy versus 42% among patients randomized to the SPECT imaging strategy (hazard ratio [HR] 0.84, 95% confidence interval [CI] [0.77 to 0.92]; p < 0.001). Overall, the trial demonstrated that SPECT MPI improved triage decision making for patients in the ED. This improvement was primarily driven by reduction in unnecessary hospitalizations among patients without acute cardiac ischemia, who comprised the majority of patients presenting to the ED.
Studies have suggested that decisions based on SPECT imaging have had cost-effectiveness implications. Although the test itself results in additional cost at the time of the ED visit, these costs are countered by a significant reduction in the frequency of unnecessary hospital admissions and overall length of stay, resulting in net savings of approximately $700 to $1900 per patient.
Based on the evidence from these two randomized trials and the numerous observational studies, the use of SPECT MPI among low-risk to intermediate-risk patients with chest pain and nondiagnostic ECG findings has since attained a Class I indication in early societal guidelines of the American College of Cardiology (ACC)/American Heart Association (AHA)/American Society of Nuclear Cardiology (ASNC) Guidelines for the Clinical Use of Cardiac Radionuclide Imaging and current Appropriate Use Criteria (AUC) consider imaging to be “appropriate” for the evaluation of chest pain using either (1) an early assessment pathway or (2) an observational pathway. The early assessment pathway is appropriate for patients who have equivocal initial troponin or single troponin elevation without additional evidence of ACS, as well as for patients with ischemic symptoms that had resolved hours before testing. This pathway includes imaging at rest (i.e., without stress testing) early on at the time of presentation to the ED, with a goal to rule in or rule out ACS or MI without waiting for serial biomarker analysis ( Table 11.1 ). Of importance, the pathway generally helps in the management and the rapid decision making in the ED but is not particularly useful in the diagnosis of any underlying CAD, particularly because it does not include stress imaging. In contrast, clinicians may pursue an observational pathway, which is appropriate among patients who undergo serial troponin testing or ECG that is either negative or borderline for NSTEMI-ACS. Unlike the early assessment pathway, the observational pathway incorporates imaging with both rest and stress testing. Based on society guidelines in emergency medicine, rest and stress MPI using the observational pathway, once serial troponin and ECG testing return negative or borderline for ACS, may even be considered on an outpatient basis within 72 hours after discharge.
| Indication | Appropriate Use Indicator |
| Diagnostic ECG for ST-elevation myocardial infarction (STEMI) | R |
| Initial history/physical examination and/or chest radiograph identifies a likely noncardiac diagnosis (pneumothorax, costochondritis, lesion of the esophagus) | R |
| Positive Initial Diagnosis of NSTEMI/ACS | |
| Initial ECG and/or biomarker analysis unequivocally positive for ischemia | R |
| Equivocal Initial Diagnosis of NSTEMI/ACS | |
| Equivocal initial troponin or single troponin elevation without additional evidence of ACS | A |
| Ischemic symptoms resolved hours before testing | M |
| Low/Intermediate Likelihood of Initial Diagnosis of NSTEMI/ACS | |
| TIMI risk score = 0; early high-sensitivity troponin negative | R |
| Normal or nonischemic on initial ECG, normal initial troponin | M |
There are several limitations to the use of radionuclide MPI in the ED setting. First, in most centers, such testing would not be available on a 24-hour basis because doing so would usually require setting up a system to have a tracer available and bringing an on-call technologist in for imaging. This concept has not been a major impediment because centers have opted to use the modality during daytime hours and at night opt for the conventional observational strategy, which is less efficient but ultimately comes to similar outcomes. Second, most image readers are comfortable assessing attenuation artifacts when reading stress and rest images together. It is more challenging to do so when only reading the rest set of images. If possible attenuation of the inferobasal wall is present, prone imaging can be used to identify attenuation artifacts ( Fig. 11.3 ). Finally, the data on the use of MPI were developed in the 1990s and early 2000s, with the major randomized trial supporting its use being published in 2002, all well before the era of high-sensitivity troponin use. A reevaluation of the data in the current era would likely demonstrate lower sensitivity overall for MPI given the new truth standard of very-high-sensitivity troponin. Nonetheless, properly targeted MPI to patients already on the lower-to-intermediate likelihood part of the risk spectrum on the basis of initial evaluation is likely to provide effective information to drive a clinician’s assessment into an even lower likelihood zone and potentially cross the comfort level threshold for a discharge decision from the ED. It is notable that the prevalence of ACS as previously noted for subjects with border zone high-sensitivity troponin (7% to 14%) is similar to the ACS prevalence in the randomized trials, such as the ERASE Chest Pain trial, so applicability of the data potentially still holds.

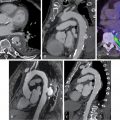
Case vignette 2: Coronary computed tomography angiography and computed tomography fractional flow reserve
A 68-year-old male presents to the ED with episodic chest pain for the past day. He has a past medical history significant for type 2 diabetes mellitus and hypertension. At the time of arrival, he is afebrile and hemodynamically stable and has a resting heart rate of 58 beats per minute. ECG shows normal sinus rhythm with no ischemic findings. A single high-sensitivity troponin I assay returns slightly elevated beyond the upper limit of normal. He is planned to undergo cardiac computed tomography angiography (CCTA) to further evaluate his coronary anatomy. Before contrast administration and image acquisition, the clinician confirms the patient has a normal renal function and a resting heart rate at goal of 50 to 60 beats per minute for optimal image quality, deferring the need for premedicating with beta-blockers. CCTA then demonstrates a focal stenosis of the proximal segment of the left anterior descending artery (LAD; Fig. 11.4 ). To further evaluate the physiologic significance of this stenosis and the potential contribution to his presenting symptoms, a noninvasive computed tomography (CT) fractional flow reserve (FFR) is calculated. FFR was 0.65 across the LAD stenosis. Because the CT-FFR is noted to be less than 0.80, the LAD stenosis is thought to be flow-limiting and the most likely cause of his symptoms. Subsequently, he is admitted and undergoes cardiac catheterization. Invasive FFR during the catheterization procedure correlates with the findings on the CT-FFR, and the patient receives one drug-eluting stent in the proximal LAD. His symptoms resolve after the intervention. The next day, he is discharged with close follow-up.