INTRODUCTION
Epidemiology
Facial fractures account for a large proportion of emergency room visits and 2% of all hospital admissions. Significant facial injuries are clinically occult in more than half of all intubated multitrauma patients. Mechanisms include motor vehicle collisions (MVCs), assault, falls, sports injuries, and civilian warfare. Together, MVCs and assault account for more than 80% of all injuries and commonly involve young adult males and alcohol use. A recent decline in MVC-related maxillofacial trauma appears to reflect improved automobile safety as a result of airbags, mandatory seatbelt laws, and improved road conditions.
Biomechanics and Associated Life-Threatening Injuries
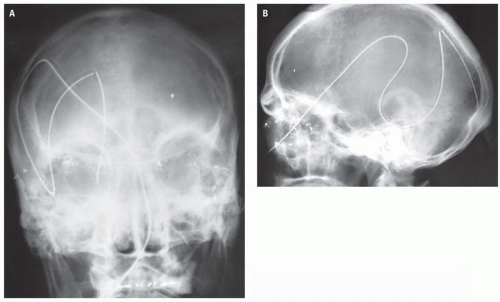
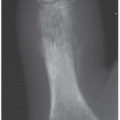
Direction and magnitude of an impacting force determines the pattern and severity of maxillofacial fractures. For example, the nose, mandibular body, and zygoma are typically injured in assault because of their prominent positions on the face and the relatively small amount of energy transferred in a strike or a punch. MVC, falls and other high-velocity injuries result in more complex, midfacial fractures. A collision of 30 miles per hour exceeds the tolerance of most facial bones (
Fig. 4.1A).
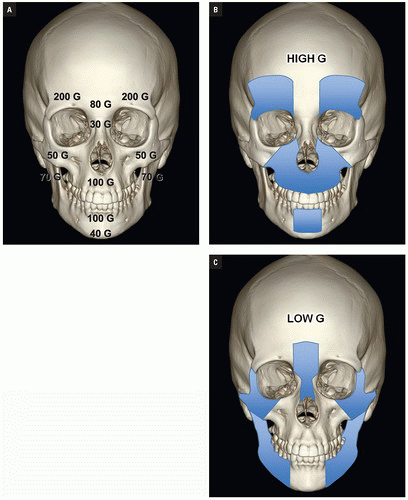
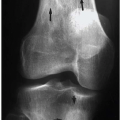
Luce et al. studied injuries associated with major facial fractures in 1,020 patients and grouped them into high and low G-force mechanisms. Life- threatening injuries included intra-abdominal injury requiring surgery, pneumothorax, chest trauma requiring ventilator support, and severe closed head injury. Twenty-one percent of patients with low G-force facial trauma had one or more of these associated injuries compared with 50% in patients with high G-force mechanisms (
Figs. 4.1B,
4.1C). Mortality in the latter group was 12%.
Mulligan et al. investigated the relationship between facial fractures, cervical spine injuries, and head injuries in 1.3 million trauma patients between 2002 and 2006. Nine percent sustained one or more facial fractures. The 6.7% of facial fracture patients had concomitant cervical spine injury, and 61.8% had associated head injury. Almost 5% suffered injuries to all three areas.
Cole et al., in a study of 247 victims of facial gunshot wounds, found associated cervical spine injury in 8% and head injury in 17%.
IMAGING
Imaging in facial trauma aims to define the number and locations of facial fractures and to identify injuries that could compromise the airway, vision, mastication, lacrimal system, and sinus function. Individual fractures should be listed and associated soft tissue injuries described with attention to these areas. If possible, bony findings should be summarized in one of several typical fracture patterns.
Imaging in most emergency departments for significant facial trauma begins with computed tomography (CT) scanning. Helical CT and, more recently, multidetector CT (MDCT) have supplanted plain radiography and have revolutionized the imaging of the maxillofacial trauma. CT is more cost efficient and more rapidly performed than radiographs of the face and mandible. MDCT is now considered the optimal imaging modality, particularly in the polytrauma setting because it allows safe and rapid image data acquisition and multiplanar reconstruction without patient manipulation. MDCT accurately depicts both bony and soft tissue injury. Submillimeter slice thickness permits exquisite multiplanar reformations (MPRs) and three- dimensional (3D) reconstructions. Fracture fragment displacement and rotation are easily determined and fracture patterns may be readily classified and assessed for stability. Volume reformations from helical and MDCT datasets enhance diagnostic accuracy and allow the surgeon to better plan operative repair by depicting complex injuries in three dimensions.
Magnetic resonance imaging (MRI) can be a useful adjunct in patients with cranial nerve deficits not explained by CT, evaluation of incidentally discovered masses, and suspected vascular dissection. Its advantages include multiplanar imaging, excellent soft tissue contrast, and lack of ionizing radiation. The practical limitations of long scan times, limited patient access, poor evaluation of bone and contraindication in patients with pacemakers, some aneurysm clips, and ocular metallic foreign bodies prevent its primary application in the emergency setting.
Multidetector Computed Tomography Technique
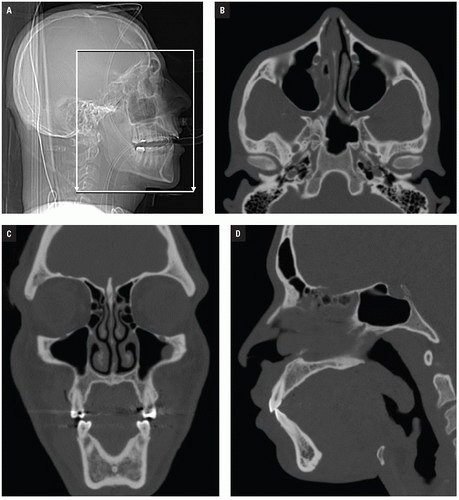
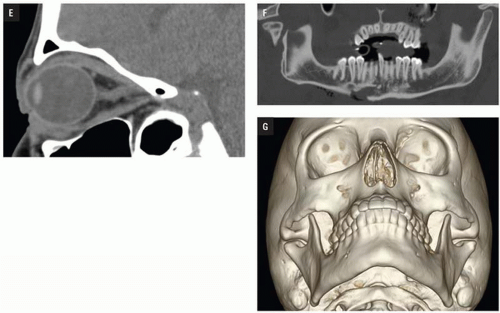
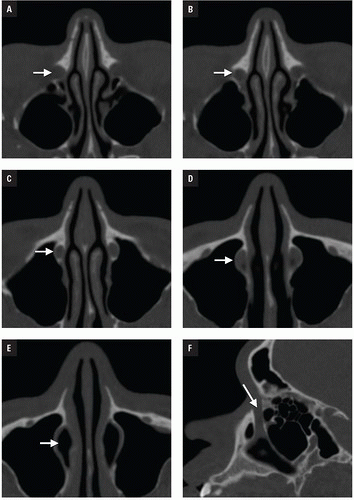
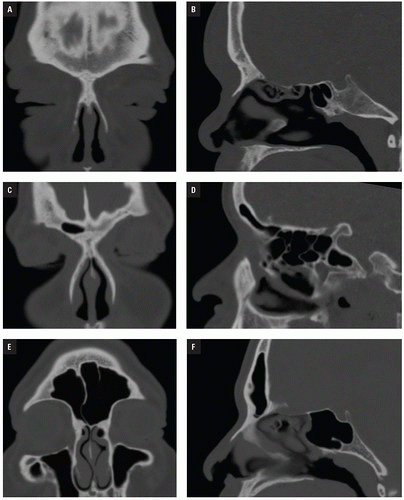
At Bellevue Hospital, patients with direct facial injury and suspected maxillofacial fractures are scanned from the hyoid through the top of the frontal sinuses. Acquisitions using 64-MDCT with 0.625-mm detector width and 0.4 mm overlapping sections allow high-quality MPRs to be generated and evaluated at the workstation. The 2 mm thick images in three planes oriented parallel and perpendicular to the hard palate provide symmetrical images for interpretation (
Figs. 4.2A-4.2D). Reconstructions in bone and soft tissue algorithm and specialized reformations may be generated depending on the presence and type of fractures. For example, oblique sagittal reformations along the plane of the optic nerve elegantly characterize orbital floor fractures with respect to depression, orbital depth, and relation to the inferior rectus muscle (
Fig. 4.2E). Panoramic or oblique sagittal planes optimize evaluation of mandibular angle and ramus fractures (
Fig. 4.2F). The 3D reconstructions can be oriented in any plane and are often acquired in patients with complex injuries as an aid to surgical planning (
Fig. 4.2G).
The multitrauma patient requires a comprehensive examination to evaluate multiple body regions in a single visit to the CT suite. With current technology, scanning of the head, face, and cervical spine may be acquired as a single acquisition and no longer requires patient repositioning for direct coronal plane imaging.
FACIAL FRACTURES
Facial fracture complexes are classified by location and pattern: nasal, naso-orbito-ethmoid (NOE), frontal sinus, orbital, zygomatic, maxillary, and mandibular. Manson et al. have proposed further categorizing each area by the energy of the injury, namely low, moderate, and high energy. Low-energy injuries show little or no comminution or displacement. Moderate-energy injuries, the most common, demonstrate mild to marked displacement, whereas high energy is reserved for cases of severe fragmentation, displacement, and instability. Impact energy subclassifications dictate management from simple closed reduction to wide exposure open reduction and internal fixation.
NASO-ORBITO-ETHMOID FRACTURES
Anatomy
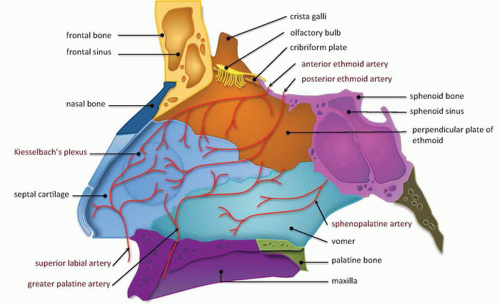
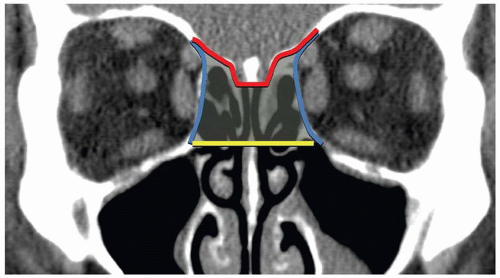
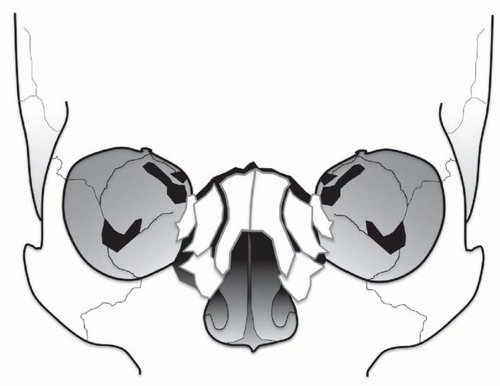
The NOE region refers to the space between the eyes or interorbital space. The interorbital space represents the confluence of the bony nose, orbit, maxilla, and cranium. It is bound laterally by the thin medial orbital walls and posteriorly by the sphenoid sinus. The cribriform plate and the medial floor of the anterior cranial fossa define its superior margin and separate the NOE region from the dura, CSF, and brain. Inferior margin is the lower border of the ethmoid air cells (
Fig. 4.6). The frontal process of maxilla, nasal process of the frontal bone, and thick proximal nasal bones comprise the anterior border of the interorbital space. Together, these form the relatively strong central facial “pillar.” Important soft tissue structures of the interorbital space include the olfactory nerves, lacrimal sac, nasolacrimal duct, ethmoid vessels, and the medial canthal ligaments.
Injuries
NOE injuries result from direct anterior impact to the upper nasal bridge and are characterized by fracture of the nasal bones, nasal septum, frontal process of the maxilla, ethmoid bones (lamina papyracea and cribriform plate), lacrimal bones, and frontal sinus (
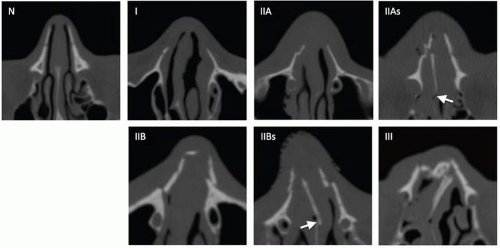
Fig. 4.7). They may be associated with other facial fractures and remote multisystem trauma. NOE fractures have been classified by Gruss who grouped them by extent of injury, displacement, orbital involvement, and associated facial fractures (
Table 4.1).
Type 1 fractures detach the frontal process of maxilla, displacing the fragments posteriorly and laterally without severe comminution. Type 2 fractures are more severely comminuted and impacted through the interorbital space, shattering the nasomaxillary buttress (discussed with maxillary fractures subsequently), and surround the piriform aperture. Subtypes a-c describe the integrity of the zygomaticomaxillary buttresses, from intact to unilateral to bilateral involvement, respectively. Type 3 fractures occur in conjunction with more extensive craniofacial injuries and reflect superolateral extension, including cribriform plate disruption with intracranial involvement and dural violation (superior extension), or LeFort II and III fractures (lateral extension). Type 4 injuries include varying degrees of orbital detachment and displacement; whereas type 5 injuries are associated with significant bone destruction or loss, potentially complicating reconstructive strategies.
The medial and lateral canthal ligaments support the globe and keep the eyelid apposed to it. Fracture through the inferomedial orbital rim suggests injury to both the medial canthal ligament and lacrimal apparatus. Patients present with nasal and periorbital ecchymosis, depression of the nasal bridge, telecanthus, enophthalmos, and a shortened palpebral fissure.
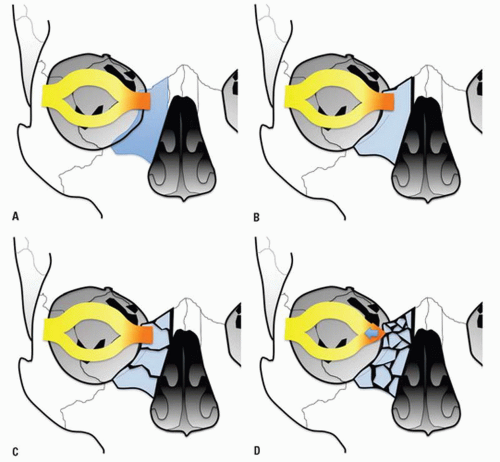
The junction of the frontal process of maxilla and the inferomedial orbital rim make up the bony anchor of the medial canthal ligament. In the setting of NOE fracture, this bony anchor is referred to as the “central” fragment and may be either intact or comminuted or fractured through the medial canthal ligament insertion site. Markowitz et al. have devised a classification system to address its integrity and dictate optimal repair (
Fig. 4.8) (
Table 4.2).
Imaging
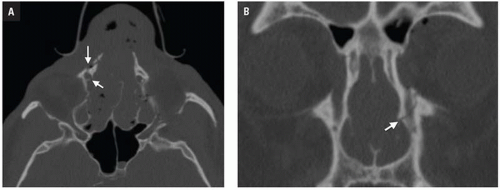
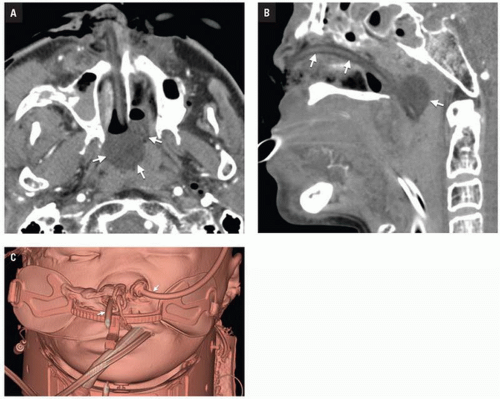
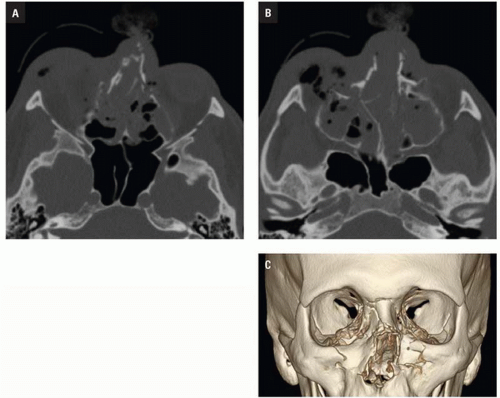
CT shows impaction of the intraorbital contents with posterior telescoping of ethmoid air cells, nasal septal buckling, and intrasinus hemorrhage. Inferomedial orbital rim fracture with displacement of the central fragment indicates medial canthal ligament involvement (
Fig. 4.9). Injuries may be unilateral or bilateral and of variable comminution depending on impact energy.
Low-energy injuries are exclusively unilateral with a single displaced inferomedial orbital rim fracture fragment. Moderate-energy NOE fractures are more common and are characterized by several fractures of the inferomedial orbital rim without fragmentation of the bony medial canthal ligament insertion. High-energy injuries disrupt the medial canthal ligament anchor and require more complex surgical repair. NOE fractures are often associated with LeFort II and III injuries and close attention should be paid to the pterygoid plates.
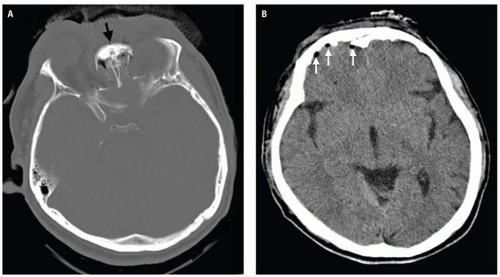
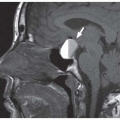
Clinical consequences include telecanthus, enophthalmos, ptosis, and lacrimal system obstruction. Associated cribriform plate fracture may result in anosmia, CSF leak, and pneumocephalus (
Fig. 4.10). The latter can evolve into tension pneumocephalus after cardiopulmonary resuscitation efforts. Disruption of the sinus roof predisposes to intracranial nasogastric tube and Foley catheter (for control of posterior nasal bleeding) malposition (
Fig. 4.11).