We present a case-based review of abdominal postoperative complications, organized by organ system affected, including wound/superficial, hepatobiliary, pancreatic, gastrointestinal, genitourinary, and vascular complications. Both general complications and specific considerations for certain types of operations are described, as well as potential pitfalls that can be confused with complications. Representative cases are shown using all relevant imaging modalities, including CT, fluoroscopy, ultrasound, MRI, and nuclear medicine. Management options are also described, highlighting those that require radiologist input or intervention.
Key points
- •
Postoperative imaging is crucial for detecting and characterizing complications in the abdomen.
- •
Many complications are common to all types of surgery, but some are specific to the type of operation performed.
- •
Radiologists must use the type of operation, acuity of presentation, indication for surgery, timing relative to operation, and presence of comorbidities to help identify and characterize complications.
- •
Radiologists must also be able to distinguish normal or expected postsurgical appearance from a complication.
In the postoperative setting, imaging plays a crucial role in detecting and characterizing complications. Many complications are common to all types of surgery, whereas others are specific to the type of operation performed. Complications vary according to the type of operation, acuity of presentation, indication for surgery, timing relative to operation, and presence of comorbidities. It is also important to distinguish normal or expected postsurgical appearance from a complication. As such, radiologists must be aware of the surgery performed and use the operative report or in-person discussion to clarify uncertainty when appropriate.
This article presents a case-based review of abdominal postoperative complications, organized by the organ system affected, including general, hepatobiliary, pancreatic, gastrointestinal, genitourinary, and vascular complications. Among these categories, specific considerations for certain types of operations are included, as well as potential pitfalls that can be confused with complications. Computed tomography (CT) is the most commonly used modality in the immediate postoperative period and/or for acutely ill hospitalized patients, but other modalities are highlighted for certain situations, including fluoroscopy, ultrasound, MR imaging, and nuclear medicine. Management options are described, highlighting those that require radiologist input or intervention.
General
Certain complications can occur after all abdominal surgeries and have a similar imaging appearance no matter the type of operation performed. These include surgical site infection, abscess, active bleeding, hematoma, and anastomotic leak (if an anastomosis has been performed). Radiologists should be familiar with the appearance of all of these but also focus their search depending on the surgery that was performed, particularly regarding the type and location of the anastomosis or anastomoses.
Wound complications include surgical site infections, seromas, hematomas, wound dehiscence, and hernias. Approximately 5% to 10% of patients who undergo major abdominal surgery will develop wound infections. Incisional surgical site infections can be superficial or deep, depending on whether the skin or subcutaneous tissue, or fascia or muscle, is infected. Both can occur within 30 days postoperatively and typically manifest with fever, peri-incisional pain, erythema, and/or purulent drainage. Diagnosis is often clinical and can be managed conservatively, but imaging can be performed when there is concern for deeper involvement or to assess for underlying fluid collections.
Soft tissue gas, nonorganized fluid, and fluid collections are common and expected postoperative findings at the incision and throughout the abdomen and pelvis. When seen on postoperative imaging, these should not necessarily be assumed to represent wound infection and should be taken in the context of the type and timing of the operation performed. As such, pneumoperitoneum in the first few days after a major operation is expected but would raise concern if increasing or persisting over time, particularly if near an anastomosis. Hemostatic material in the operative bed or packing material in a superficial wound can also mimic an infected fluid collection ( Fig. 1 ). Moreover, imaging alone cannot include or exclude the presence of infection in a postoperative collection but should be used to document the size and extent. A superficial or intraabdominal collection that becomes more well-organized, particularly with increasing internal gas and rim enhancement, should be considered highly suspicious for abscess when there are concurrent clinical signs of infection ( Fig. 2 ). When abscess is suspected, percutaneous aspiration and/or drainage should be performed to confirm the diagnosis.


Hematomas are the most common superficial postoperative collection and can occur as a result of incomplete hemostasis and/or bleeding diathesis ( Fig. 3 ). No matter their location, all hematomas have similar imaging findings: high attenuation on CT, heterogeneously echogenic on ultrasound, and high signal on T1-weighted imaging ( Fig. 4 ). On CT, the attenuation will vary depending on the composition of the hematoma. Clotted blood is 45 to 70 HU, whereas unclotted blood is 30 to 45 HU, which can result in the hematocrit effect in which higher attenuation layers along the dependent portion of the hematoma. After laparoscopic procedures, abdominal wall hematomas can occur secondary to trocar insertion and damage to the small vessels in the abdominal wall. Intraabdominal hematomas usually occur in the surgical bed, near the vascular anastomosis (if performed) or in dependent spaces, and normally resolve spontaneously within a few weeks. Multiphase CT can demonstrate active bleeding by showing intravenous contrast extravasation into a hematoma.


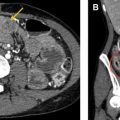
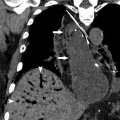
Other vascular complications include thrombosis, pseudoaneurysm, stenosis, and infarction, and can be seen after any type of surgery, though the risk is higher with increasing complexity or acuity of the operation. Pseudoaneurysms should be considered whenever there is an apparently cystic structure near an arterial anastomosis. Pseudoaneurysms occurring after biopsy or other percutaneous interventional procedure may also be seen within the parenchyma. Doppler ultrasound will demonstrate bidirectional flow at the neck, whereas CT or MR angiography will show a saccular lesion enhancing with the blood pool. Embolization and/or stent placement are usually performed to prevent rupture. Biliary or gastrointestinal tract fistulas can also result from pseudoaneurysms.
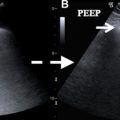
A thrombosed artery or vein will demonstrate absent flow with an echogenic filling defect on ultrasound, whereas CT and MR imaging depict a filling defect. For superficial venous thrombus, lack of compressibility on ultrasound may be the earliest finding as the actual thrombus is isoechoic to blood. Stenosis on ultrasound manifests as focal color aliasing, poststenotic dilation, and manifestations of portal hypertension (for portal vein stenosis). After liver transplant, the normal portal vein commonly has reduced caliber at the anastomosis (reflecting a size discrepancy between donor and recipient) with turbulent flow, but this does not indicate stenosis.
Seromas are superficial or intraabdominal collections of serous fluid that typically have simple fluid attenuation and commonly resolve by resorption. Although most small collections spontaneously resolve, they can predispose to superinfection and wound dehiscence if they persist. As such, any collection can be aspirated or drained prophylactically to prevent subsequent infection and dehiscence, especially when present in large dead spaces or in high-risk patients. Wound dehiscence can involve a portion or the entire incision. CT findings include separation of the abdominal wall layers, commonly with interspersed fluid or gas ( Fig. 5 ).

Incisional hernias are more common with vertical than horizontal incisions, occurring in 5% to 15% of open procedures and 1% to 3% of minimally invasive procedures. Incisional hernias are commonly managed expectantly until there are significant symptoms or concern for incarceration. Imaging can be used to confirm the diagnosis, particularly to distinguish a hernia from diastasis and to assess for concurrent findings of bowel obstruction. Ultrasound can be used to diagnose or confirm small hernias, but CT is most frequently used for preoperative planning. In this context, radiologists should describe the size, location, and type of the incisional hernia, as well as the presence of fluid collections, mesh, or bowel, within the defect, including specific types of hernias ( Fig. 6 ).

Prosthetic mesh is used for most incisional hernia repairs and infection of the mesh is a specific postoperative complication that reportedly occurs in 0.7% to 1.9% of patients. Use of concurrent immunosuppression, urgent repair, and postoperative surgical site infection are predictors of mesh infection. Most meshes are synthetic or biologic and can be placed intraperitoneal (between the peritoneum and greater omentum) or retromuscular (between the muscle and its posterior aponeurosis). On CT, the appearance is variable but, often, thin curvilinear structures that are iso-attenuation or high attenuation relative to the adjacent muscle. Infected mesh with abscess is the most feared complication because it almost always requires reoperation with mesh removal, debridement of surrounding tissue, and repair of the abdominal defect (either immediately or in a delayed fashion), which can be a very challenging operation. When infection is suspected on CT, it is important to describe the location of the fluid or fluid collection relative to the mesh and abdominal wall musculature in order to facilitate percutaneous aspiration and surgical planning.
Surgical anastomoses can be vascular, biliary, enteric, or urinary, all of which can be complicated by dehiscence and leaking. Nonorganized fluid, gas, edema, and fat stranding around an anastomosis are common postoperative findings and confirming the presence of a leak often requires visualizing extraluminal contrast in the operative bed. Nondistended bowel loops are common pitfalls that can be mistaken for fluid collections. The different types of anastomoses may be best evaluated with specific types of contrast (eg, enteric, biliary, urinary) and attention to certain locations (see later discussion).
Pitfalls
- •
Expected postoperative fluid and gas
- •
Hemostatic material containing gas
- •
Portal vein size discrepancy between donor and recipient
- •
Diastasis
- •
Wound healing by secondary intention ( Fig. 7 )

Fig. 7
A 55-year-old man was transferred from Mexico after numerous abdominal surgeries due to small bowel obstructions, which were subsequently complicated by enterocutaneous fistulas. Reportedly, the wound could not be closed at the outside hospital due to infection and was left open to heal by secondary intention. 3D reformatted image ( A ) and axial CT image ( B ) of the mid abdomen demonstrate a 12 cm by 15 cm open wound ( arrows ) with bowel and mesenteric vasculature near the surface.
- •
Nondistended bowel loops mimicking fluid collection.
Hepatobiliary
Hepatobiliary complications most commonly occur after hepatic resection, cholecystectomy, liver transplant, and liver biopsy but can also occur after total and partial gastrectomy due to the proximity of the stomach to the liver. Potential complications include bile duct injury, bile leak, biliary ischemia, thrombosis, arterial stenosis, and hepatic infarct.
Iatrogenic biliary injury is the most feared complication after cholecystectomy, particularly if it involves the common bile duct (CBD). CBD injury is widely reported at 0.4% to 0.6% in laparoscopic cholecystectomy compared with 0.2% to 0.3% after open cholecystectomy, although more recent reports suggest that the rates may be similar as laparoscopic experience becomes more uniform. Of these, cystic duct stump leak is the most common source after laparoscopic cholecystectomy. Bile leaks also complicate 2% to 25% of liver transplants or hepatic resection, often at sites of biliary anastomosis or from the cut liver surface, and are the second-most-common cause of graft dysfunction after liver transplant. Similarly, CBD injuries are very important to recognize and manage because they have a devastating impact on morbidity and mortality for patients after cholecystectomy, with an 8.8% increase in all-cause mortality compared with patients without CBD injury, and 5-year mortality rates exceeding 20%. The risk of iatrogenic CBD injury significantly increases when patients present with acute cholecystitis or have variant anatomy. If unrecognized intraoperatively, patients with biliary injuries typically present within 1 week with symptoms of bile leak or stasis, such as abdominal pain, fever, nausea, and hyperbilirubinemia.
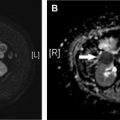
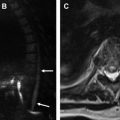
Imaging evaluation can be performed with ultrasound, CT, MR cholangiopancreatography (MRCP), or hepatobiliary iminodiacetic acid (HIDA) scan, with the choice determined by the acuity of presentation and level of suspicion. Ultrasound is useful for documenting the presence of a fluid collection and biliary dilation but is unlikely to identify to site of injury. CT can demonstrate similar findings, as well as the extent of fluid collections or peritoneal inflammation and concomitant vascular injuries. MRCP is very accurate for identifying the exact site of biliary injury and distinguishing between biliary and nonbiliary fluid collections, particularly when a hepatobiliary contrast agent is used (eg, gadoxetic acid [Gd-EOB-DTPA]) ( Fig. 8 ). Compared with endoscopic retrograde cholangiopancreatography, MRCP also has the advantage of delineating anatomy proximal and distal to the injury, including discontinuous ducts, as well as the patency of biliary-enteric anastomosis, which is important for treatment planning ( Fig. 9 ). MRCP is also the best imaging modality for assessing for postoperative biliary stricture, including in the setting of biliary-enteric anastomosis ( Fig. 10 ). HIDA is the most sensitive test for detecting a bile leak and can help demonstrate contained versus free leakage. It historically has lacked the spatial resolution to localize the exact site of injury, although single-photon emission CT is now commonly used with improved specificity ( Fig. 11 ). Notably, postoperative fluid collections and gas are commonly detected and are not specific for a biliary injury.





Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree