Neuroimaging is an invaluable diagnostic tool for sorting through the vast array of etiologies that underlie altered mental status (AMS). Head computed tomography (CT) without contrast is the primary modality for evaluation of AMS and should be complemented by MR imaging in cases of negative CT but high clinical concern. Studies to maximize brain imaging efficiency and improve the yield of positive scans through the utilization of clinical and laboratory pre-scan diagnostics are ongoing. However, imaging remains the gold standard due to its rapidity with which certain diagnoses can be made or excluded.
Key points
- •
The clinical history of altered mental status implies a broad differential, and can be a challenge to the managing physicians. It refers to either disturbed consciousness or decreased arousal.
- •
There are numerous etiologies underlying altered mental status, either related to systemic illnesses or central nervous system processes (organic or psychiatric).
- •
Neuroimaging is an important tool for solving the clinical dilemma and gleaning through the vast array of etiologies of consciousness perturbations.
- •
Given the serious consequences of a delay in diagnosis, obtaining brain imaging early is crucial.
- •
Noncontrast computerized tomography of the brain provides a rapid screening, whereas MR imaging is reserved for more complex presentations unsolved by CT.
Introduction
Altered mental status (AMS) is one of the most common indications for brain imaging of both emergency room and hospitalized patients. AMS is a broad term, and can imply either changes in consciousness (supratentorial function) or in arousal (executed by the brainstem). Underlying etiologies includes both systemic illnesses and central nervous system processes, the latter encompassing both organic and psychiatric causes. The long and varied differential diagnosis can make AMS a potentially vexing clinical problem.
AMS (or encephalopathy) can be generally divided into the following 2 broad categories:
- •
S ystemic etiologies, such as infections, metabolic derangements, side effects of medications, vasculitis, autoimmune encephalitis, and, at our institution, polysubstance abuse.
- •
O rganic brain causes, such as new or worsening intracranial hemorrhage, traumatic brain injury, central nervous system infections, mass effect/herniation related to a tumor or to new/increasing size of an infarction, reversible posterior encephalopathy syndrome, nonconvulsive seizures, and acute hydrocephalus.
AMS is often an acute or subacute event, and the timely identification of potentially treatable intracranial pathology is paramount to its management. Several nonimaging diagnostic tests have been proposed to predict imaging findings, but their utility is generally limited due to the urgency of the clinical situation. In mild traumatic brain injury, serum biomarkers, such as glial fibrillary acidic protein and ubiquitin C-terminal hydrolase-1, have been established as predictors of intracranial hemorrhage. In atraumatic scenarios, various tentative criteria have been advocated as foretelling of a positive brain scan, such as elevated diastolic blood pressure, focal weakness, low Glasgow Coma Scale score, antiplatelet/anticoagulant use, plantar reflexes, presence of headache, dilated pupils, and low C-reactive protein.
Neuroimaging, particularly noncontrast head computed tomography (CT), provides the most rapid and effective screening tool for AMS. Although sometimes criticized for its “overuse,” the gravity of delay leaves managing physicians little leeway to forfeit brain imaging.
Given the breadth of the subject, this article focuses on some common clinical problems and a select group of interesting presentations underlying AMS. Related clinical diagnoses and indications for neuroimaging are further discussed in the Judith Ann Gadde and colleagues’ article, “ Neuroimaging of Patients in the ICU: Pearls and Pitfalls ,” in this issue.
Hypoxic-anoxic brain injury, also known as hypoxic-ischemic brain injury
Encephalopathy due to hypoxic-anoxic brain injury, also known as hypoxic-ischemic brain injury (HIBI), is the result of decreased perfusion and oxygenation of the brain and is due to hypotension/hypoperfusion from cardioembolic phenomenon or drastically decreased cardiac output.
The leading etiology of HIBI is cardiac arrest (also known as pulseless electrical activity, PEA arrest). Interestingly, these patients with cardiac arrest are most likely to die because of the hypoxic-anoxic brain injury (HIBI is the primary cause of death in 68% of inpatient cardiac arrests).
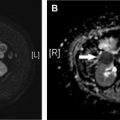
The spectrum of imaging findings of HIBI is broad, with appearance from subtle to striking. In the hyperacute injury, the head CT findings can be nearly imperceptible ( Fig. 1 A), and clinical history is important. Conversely, the HIBI is sometimes not suspected clinically and is apparent on imaging.
Mild Hypoxic-Anoxic Injury
Symptoms and imaging findings can be reversible in milder cases, particularly if the gray matter (basal ganglia and cortex) is spared. The patients who develop only early supratentorial deep white matter involvement ( Fig. 1 B) have a better prognosis and the imaging findings mirror the clinical course. A delayed post-hypoxic leukoencephalopathy has also been described, and patients could re-present with symptoms 2 to 3 weeks after an initial recovery.
Infratentorial involvement in HIBI ( Fig. 1 C) is much less common. The cerebellar white matter symmetric involvement has been usually associated with toxic-metabolic insults and is seen with spongiform leukoencephalopathy related to illicit drug use such as heroin, and particularly with inhalation of heated heroin vapors (also known as “chasing the dragon”). The imaging diagnosis challenge lies in discerning between HIBI and toxic-metabolic injury.
Severe Hypoxic-Ischemic Injury
In severe hypoxic-ischemic injury, the brain injury is due to decreased blood flow and impaired removal of harmful cellular metabolites, such as lactate and glutamate, which accumulate and have deleterious effects.
The severe HIBI can result in florid edema ( Figs. 2 and 3 ), causing hyperdense appearance of the subarachnoid space, and can eventually result in downward brain herniation (see Fig. 3 C). Of course, the prognosis is guarded.


At our institution, many of the HIBI cases are imaged with MR imaging in the delayed subacute stages, when the lack of clinical improvement becomes evident. These patients are not regaining consciousness as expected after treatment of other conditions for which they were admitted. Delayed MR imaging diagnosis can be a challenge, as the acute findings of cytotoxic edema (reduced diffusion and T2 hyperintensity of the basal ganglia and cortex) subside and only subtle residua can be perceived. Arterial spin labeling perfusion and MR spectroscopy may be helpful in some cases.
Hypoglycemia
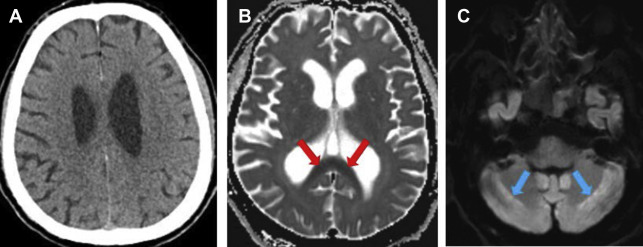
Hypoglycemia is well-known to cause central nervous system sign and symptoms, from focal neurologic deficits to coma and death, and its imaging manifestations can mirror the clinical severity. Characteristic involvement of the hippocampus, cerebral cortex, and basal ganglia with sparing of the posterior fossa has been described on MR imaging. Isolated deep supratentorial white matter involvement also can occur ( Fig. 4 A, B ).

With profound hypoglycemia, cerebral fullness can develop due to vasogenic edema related to decreased osmolality of the parenchyma. The blood-brain barrier has been demonstrated to remain intact. The CT appearance may be confused with hypoxic-anoxic injury or edema related to traumatic brain injury. The distinction can be made by the observation that even though the cerebrospinal fluid (CSF) spaces can be completely effaced, the gray-white differentiation remains preserved in hypo-osmolality-induced cerebral edema ( Fig. 4 C, D).
Teaching point
Sulcal and ventricular effacement can be related to markedly decreased cerebral parenchymal osmolality, such as seen with profound hypoglycemia, hyponatremia, or with volume overload (ie, polydipsia). The imaging findings are usually reversible, the gray-white differentiation is preserved, and the appearance should not be confused with hypoxic-anoxic or traumatic brain injury.
Hydrocephalus
There are several etiologies for the development of acute hydrocephalus, such as meningitis, subarachnoid hemorrhage from aneurysmal rupture, enlarging lesions producing mass effect at the foraminae (of Monro, of Luschka or Magendie, magnum), fourth ventricle, or Sylvian aqueduct ( Fig. 5 ). The compressive effect of the enlarged ventricles on the adjacent parenchyma causes interstitial edema and axonal hypoxia and results in subsequent retrograde neuronal damage, which is thought to be responsible for the alteration in level of consciousness. A dysregulation due to altered innervation or disruption of local cell interactions has also been implicated.


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree