Grade
Description
I
Hematoma: subcapsular, <10 % surface area
Laceration: capsular tear, <1 cm in parenchymal depth
II
Hematoma: subcapsular, 10–50 % surface area; intraparenchymal, <10 cm in diameter
Laceration: 1–3 cm in parenchymal depth, <10 cm in length
III
Hematoma: subcapsular, >50 % surface area or expanding or ruptured subcapsular parenchymal hematoma; intraparenchymal hematoma >10 cm or expanding or ruptured
Laceration: >3 cm in parenchymal depth
IV
Laceration: parenchymal disruption involving 25–75 % hepatic lobe or 1–3 Couinaud segments
V
Laceration: parenchymal disruption involving >75 % of a hepatic lobe or >3 Couinaud segments within a single lobe
Vascular: juxtahepatic venous injuries (i.e., central major hepatic veins or retrohepatic vena cava)
VI
Vascular: hepatic avulsion
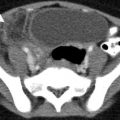
Hepatic injuries detected by CT can be classified as lacerations, hematomas, active hemorrhage, and juxtahepatic venous injuries. Hepatic laceration is the most common type of parenchymal liver injury; it appears as an irregular, linear, or branching low-attenuation region on contrast-enhanced CT (CECT) (Fig. 9.1). Lacerations are further divided into superficial (<3 cm) or deep (>3 cm).
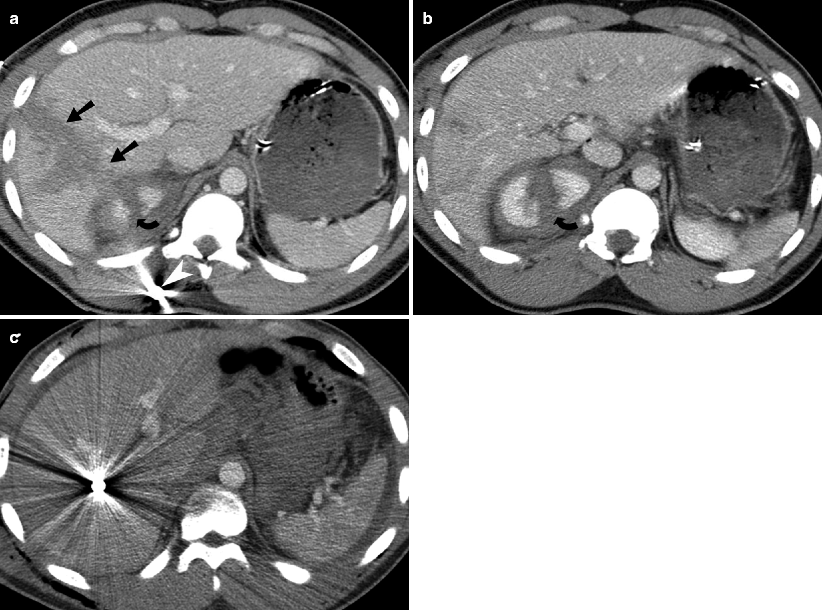

Fig. 9.1
Hepatic laceration from gunshot wound. (a and b) Contrast-enhanced CT demonstrates a deep hepatic (straight arrow) as well a right renal laceration (curved arrow). There is a bullet fragment (white arrowhead) located in the right posterior abdominal wall. (c) Contrast-enhanced CT demonstrates a metallic density bullet fragment located centrally with in the right lobe of liver. The liver laceration is obscured by the extensive beam-hardening artifact produced by the bullet fragment
Hematomas that present in blunt liver trauma are designated as subscapular or intraparenchymal. On CECT, a subcapsular hematoma appears as an elliptical collection of low-attenuation blood between the capsule of the liver and the enhancing liver parenchyma (Fig. 9.2). Intraparenchymal hematomas are characterized by focal low-attenuation regions with poorly defined, irregular margins in the liver parenchyma on CECT (Fig. 9.1). Active hemorrhage is diagnosed by identification of a focal high-attenuation area representing a collection of extravasated contrast. Active vascular extravasation can often be differentiated from clotted blood by measuring the CT attenuation coefficient. The attenuation of clotted blood ranges from 28 to 82 Hounsfield units (HU) (mean, 54 HU), whereas active arterial extravasation ranges from 91 to 274 HU (mean, 155 HU) [10]. Active contrast extravasation (ACE) changes its appearance over time; such a pattern can be demonstrated with multiphase vascular imaging, that is, during the arterial, portal venous, or delayed phases. On later vascular phase imaging, a region of ACE will increase in size and often pool or mix with non-contrasted blood in the adjacent hematoma.
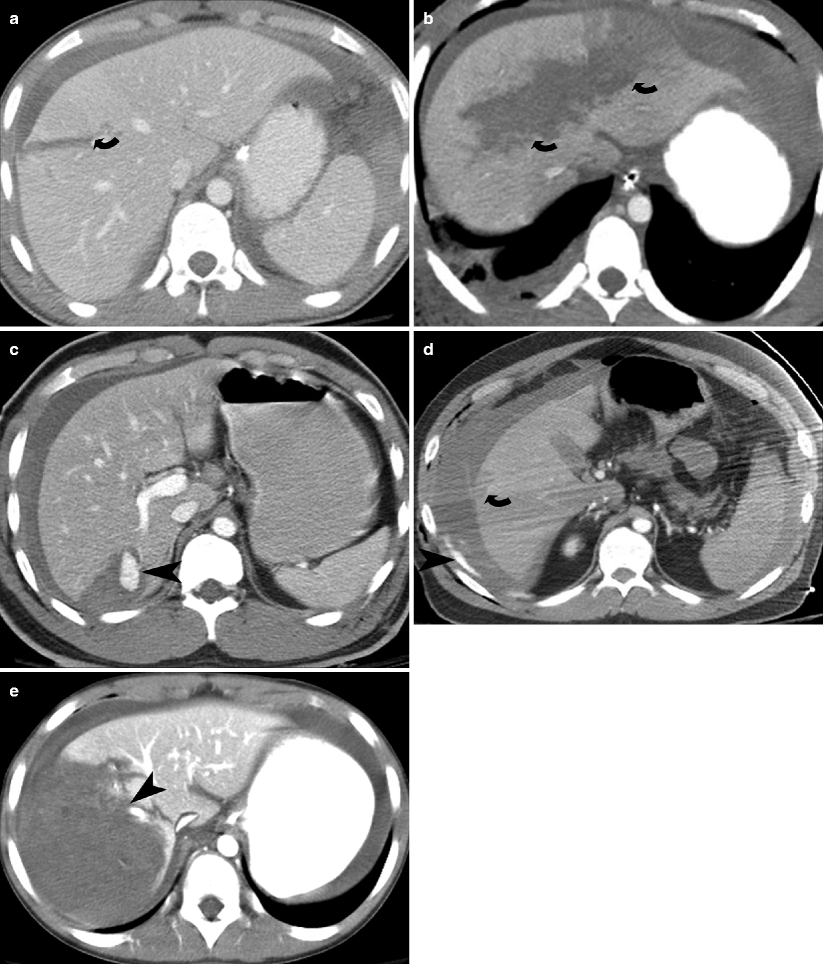

Fig. 9.2
Hepatic lacerations. (a and b) Contrast-enhanced CT in two different patients show a deep (>3 cm) hepatic laceration (grade III and grade IV liver injury) (curved arrows) due to stab injury. (c) Contrast-enhanced CT shows a wedge shaped liver laceration due to stab injury with subcapsular hematoma and enhancing pseudoaneurysm (arrowhead). (d) Contrast-enhanced CT shows a large perihepatic hematoma and active extravasation (arrowhead) arising from a gunshot injury related superficial liver laceration (curved arrow). (e) Contrast-enhanced CT shows grade IV liver injury with devascularization of >25 % of the right lobe of the liver (arrowhead)
Hepatic lacerations or hematomas that extend into a major venous structure indicate a severe injury and have been reported to require surgical management approximately 6.5 times more frequently than injuries not involving the hepatic veins or inferior vena cava (IVC) [11]. A CT finding that may indicate liver injury is periportal low attenuation paralleling the portal vein and its branches. Periportal low attenuation adjacent to a hepatic laceration may represent extension of hemorrhage into the periportal connective tissue, although this finding is nonspecific. It can also represent distention of the periportal lymphatic vessels as can be seen after aggressive fluid resuscitation, tension pneumothorax, or pericardial tamponade [12].
Spleen
Currently, 60–80 % of patients who sustain blunt splenic injury are managed nonoperatively with a success rate near 95 % [2]. Nonoperative management of isolated splenic injury is contingent on hemodynamic stability. Inevitably, failure of nonoperative management correlates with the presence of ACE on CT scan as well as with the radiological grade of the injury per the AAST criteria [9] (Table 9.2).
Table 9.2
AAST organ injury scale for spleen
Grade | Description |
|---|---|
I | Hematoma: subcapsular, <10 % surface area |
Laceration: capsular tear, <1 cm in parenchymal depth | |
II | Hematoma: subcapsular, 10–50 % surface area; intraparenchymal, <5 cm in diameter |
Laceration: capsular tear, 1–3 cm in parenchymal depth, not involving a trabecular vessel | |
III | Hematoma: subcapsular, >50 % surface area or expanding; ruptured subcapsular or parenchymal hematoma; intraparenchymal hematoma >5 cm or expanding |
Laceration: >3 cm in parenchymal depth or involving trabecular vessels | |
IV | Laceration: involving segmental or hilar vessels producing major devascularization (>25 % of spleen) |
V | Hematoma: completely shattered spleen |
Laceration: hilar vascular injury that devascularizes spleen |
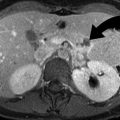
CECT can accurately diagnose the four common types of splenic injury: hematoma, laceration, active hemorrhage, and vascular injuries [13]. Splenic hematomas may be classified as subcapsular or intraparenchymal. On CECT, a subcapsular hematoma appears as an elliptical, low-attenuating collection between the splenic capsule and the enhancing splenic parenchyma (Fig. 9.3). Acute lacerations have a jagged or sharp margin and appear on CECT as a linear or branching low-attenuation area.
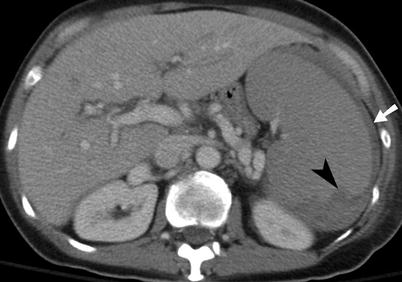

Fig. 9.3
Splenic laceration. Contrast-enhanced CT shows subcapsular hematoma (arrow) and intraparenchymal laceration (arrowhead) with hematoma
Active hemorrhage in the spleen is represented as an irregular or linear focus of contrast extravasation on CECT. Active hemorrhage may be seen in several locations: within splenic parenchyma or subcapsular space or intraperitoneally. Differentiating between ACE (range 85–350 HU, mean 132 HU) and hematoma or clotted blood (range 40–70 HU, mean 51 HU) is accomplished by measuring the attenuation coefficient [13].
Splenic vascular injuries include post-traumatic pseudoaneurysms and arteriovenous (AV) fistulas. A splenic pseudoaneurysm will appear as a well-circumscribed focus of increased attenuation in comparison to the enhancing splenic parenchyma (Fig. 9.4). An AV fistula is best demonstrated by early splenic vein enhancement. Both of these vascular injuries are best seen on arterial phase imaging and can be difficult to detect on portal venous phase or delayed (renal excretory phase) imaging. If a splenic pseudoaneurysm is suspected on early arterial phase imaging, it is helpful to distinguish this finding from ACE by noting the characteristics on delayed imaging. Specifically, on delayed imaging, a pseudoaneurysm will remain the same size and demonstrate similar density to the aorta, but ACE will increase in size and remain with high density. Splenic vascular lesions can be managed successfully by splenic arteriographic embolization, which improves the success rate of nonoperative management of blunt splenic injuries from 87 to 94 % [14, 15].
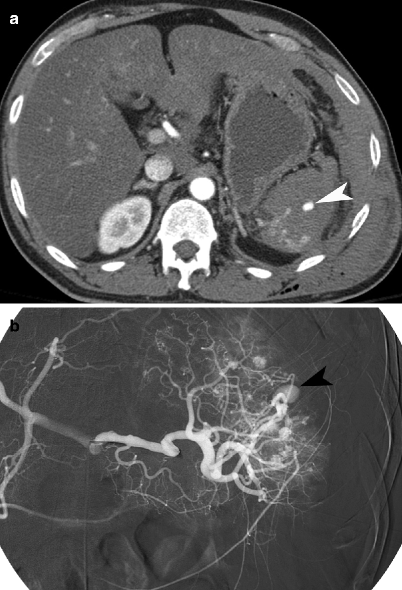

Fig. 9.4
Splenic pseudoaneurysm. (a) Contrast-enhanced CT demonstrates a pseudoaneurysm (arrowhead) within the splenic parenchymal laceration. (b) Conventional splenic artery angiogram demonstrates the splenic arterial branch pseudoaneurysm (arrowhead)
Pancreas
Pancreatic injuries have been reported as high as 12 % in victims of blunt trauma and 6 % in those with penetrating trauma. Typically, pancreatic injuries are associated with other intra-abdominal injuries 50–98 % of the time [13]. The clinical diagnosis of pancreatic injury may be difficult, particularly when isolated. Owing to the retroperitoneal location of the pancreas, peritonitis from a pancreatic injury may take hours to days to manifest. In addition, serum and urinary amylase levels are unreliable markers for the diagnosis of pancreatic injury [16].
CECT is the modality of choice for diagnosing pancreatic injury; its reported sensitivity and specificity is as high as 85 % [17]. CECT findings of pancreatic injury may be subtle, and the pancreas may appear normal immediately post-injury. Of primary importance is evaluation of the pancreatic duct because its integrity or lack of integrity directs management.
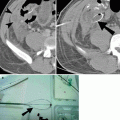
A pancreatic injury can be categorized as contusion, laceration, or transection. A pancreatic contusion may appear as diffuse enlargement of the pancreas or as focal low attenuation or heterogeneity. Pancreatic lacerations are demonstrated by linear, irregular low-attenuation areas within the normally enhancing parenchyma (Fig. 9.5). A pancreatic transection may be difficult to diagnose with CT unless there is low-attenuation fluid collection separating the two edges of the transected pancreas.
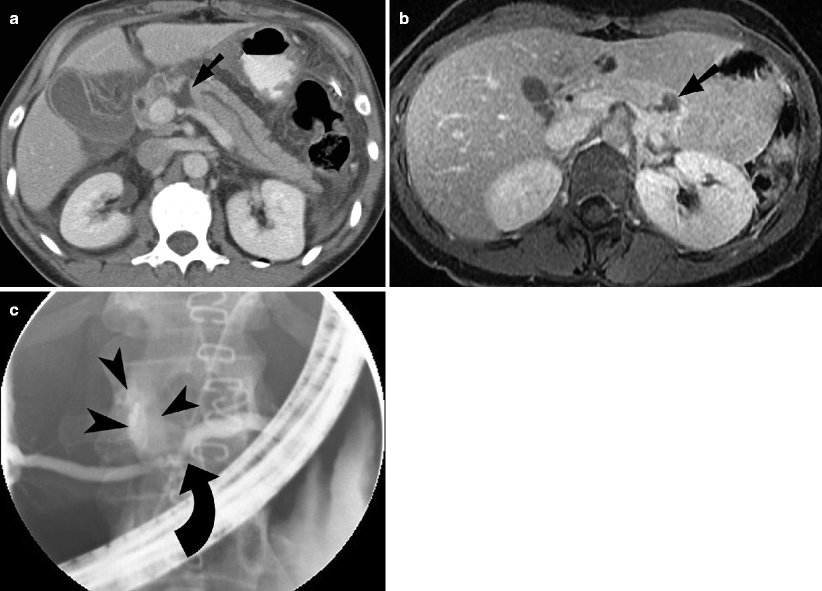

Fig. 9.5
Pancreatic injury. (a and b) Contrast-enhanced CT demonstrates pancreatic laceration (arrow) with disruption of the main pancreatic duct. (c) Endoscopic retrograde cholangiopancreatography image showing pancreatic duct discontinuity (curved arrow) and extravasation of contrast from the ductal disruption (arrowheads)
The position of the pancreatic laceration in relation to the superior mesenteric artery as well as the depth of the laceration helps predict pancreatic ductal disruption, which occurs in up to 15 % of pancreatic trauma [13, 17]. The superior mesenteric vessels provide a landmark for dividing the pancreas into proximal and distal portions with injury to the proximal pancreas usually associated with more severe injury. A laceration of the pancreas involving >50 % of the anteroposterior diameter of the pancreatic body or tail is often associated with ductal disruption.
There are several nonspecific CT findings associated with pancreatic trauma, the most common of which is thickening or infiltration of the anterior pararenal fascia. Additional nonspecific CT findings include blood/fluid tracking along the mesenteric vessels, fluid in the lesser sac, fluid between the pancreas and splenic vein, or infiltration of the peripancreatic fat with fluid or hemorrhage [13].
Kidney
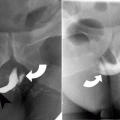
The kidney is the most commonly injured urogenital organ in trauma. Approximately 10 % of all significant blunt abdominal traumatic injuries include a renal injury, and of those, 80–90 % are managed nonoperatively. The goal of conservative management is to preserve organ integrity and reduce the complication rate. Historical evidence shows that hemodynamically stable patients with kidney injuries who undergo surgical exploration have a much higher incidence of nephrectomy [4]. Blunt trauma accounts for approximately 90 % of renal trauma, while penetrating trauma accounts for approximately 10 %. Nonsurgical management is more commonly advocated in blunt renal injuries, but conservative protocols have also been applied to penetrating renal injuries [18, 19]. However, penetrating trauma is more frequently associated with major renal injury and frequently requires invasive treatment, as it is more often associated with hemodynamic instability and damage to surrounding abdominal organs [20]. Indications for renal imaging include gross hematuria and penetrating or blunt trauma with hematuria. The imaging modality of choice to evaluate the kidneys after trauma is CECT.
Renal injuries may be classified as lacerations, contusions, or renovascular injuries, which determine the radiological grade of the injury per AAST criteria (Table 9.3). Renal contusions are visualized as poorly marginated, round or ovoid areas of low-attenuation and show a delayed or persistent nephrogram when compared to normal adjacent renal parenchyma. Hematomas can be categorized as subcapsular or perinephric. On an unenhanced CT, a subcapsular hematoma (Fig. 9.6) is seen as an eccentric hyperattenuating fluid collection confined between the renal parenchyma and renal capsule. However, on a CECT a subcapsular hematoma will be hypoattenuating compared to the normal enhancing renal parenchyma. A subcapsular hematoma may also exert a mass effect on the renal contour and can cause decreased perfusion in extreme cases. A perinephric hematoma is a poorly marginated, hyperattenuating fluid collection (45–90 HU) that is confined between the renal parenchyma and the Gerota fascia [21]. Other findings associated with a perinephric hematoma are thickening of the lateroconal fascia, compression of the colon, and displacement of the kidney.
Table 9.3
AAST organ injury scale for kidney
Grade | Description |
|---|---|
I | Hematoma: subcapsular, nonexpanding without parenchymal laceration |
Contusion: microscopic or gross hematuria, urologic studies normal | |
II | Hematoma: nonexpanding perirenal hematoma confirmed to renal retroperitoneum |
Laceration: <1 cm parenchymal depth of renal cortex without urinary extravasation | |
III | Laceration: >1 cm in parenchymal depth of renal cortex without collecting system rupture or urinary extravagation |
IV | Laceration: parenchymal laceration extending through renal cortex, medulla, and collecting system |
Vascular: main renal artery or vein injury with contained hemorrhage | |
V | Hematoma: completely shattered kidney
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|