Imaging of Brain Trauma
Keith J. Smith
Diane Armao
Mauricio Castillo
NORMAL ANATOMY
Brief Overview, Computed Tomography
Computed tomography (CT) is the most commonly used imaging method in the patient with acute neurological deficits, acute onset of severe headache, or cranial trauma. The examinations are generally obtained using 3 to 5 mm thick sections in the axial plane. We now use spiral (helical) technique for all trauma brain CT studies. These are acquired by 0.6 mm slice thickness (pitch = 1) and reconstructed by 4 or 5 mm slice thickness. Examinations performed for trauma or acute mental status changes generally do not require contrast administration. The images are obtained beginning at the base of the skull and extending to the vertex. Generally, the images are presented with soft tissue window settings (width: 110 to 120 for the posterior fossa and 80 for the cerebral hemispheres, level: 40) and bone window settings (width: 3,500, level: 700). The so-called “blood” or intermediate windows (width: 250, level: 80) are helpful for identification of small extra-axial (between the brain and skull) collections of acute blood. The normal gray matter measures approximately 25 to 40 Hounsfield units and the normal white matter approximately 25 to 30 units. In newborns and young children, the white matter is relatively hypodense compared to that of adults.
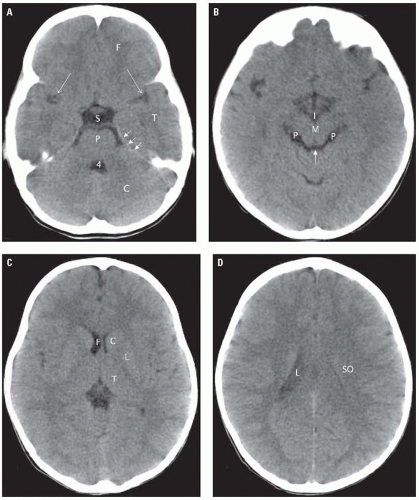
The normal fourth ventricle appears in the midline as an inverted U-shaped structure (Fig. 2.1A) which may contain calcified choroid plexus. In older individuals, the perimedullary, prepontine, and cerebellopontine angle cisterns are clearly seen and are low (fluid) density (Fig. 2.1B). All cerebrospinal fluid (CSF) containing spaces have a CT density of 0 to 20 units. The tentorium cerebelli separates the infratentorial and supratentorial compartments.
The tentorium is slightly denser than the brain and has an inverted V shape. This structure may be difficult to visualize in the absence of calcification, contrast administration, or acute blood layering on it. Structures lateral to the tentorium are supratentorial, while all structures medial to it are infratentorial in location. The interpeduncular cistern is located at the level of the midbrain (Fig. 2.1B). This is an important landmark because subarachnoid hemorrhage (SAH) tends to accumulate in this location. Anterior to this cistern is the suprasellar cistern. This CSF-filled structure has either a five-point or six-point appearance. Its borders are formed anteriorly by the gyri recti, laterally by the temporal lobe unci, and posteriorly by the cerebral peduncles. The most important structures contained in the suprasellar cistern are the optic chiasm and the apex of the basilar artery. The Sylvian fissures are generally symmetrical and of CSF density. Medial to these fissures are the insulae—subinsular regions (which contain the extreme capsules, claustra, and external capsules). The structures within the subinsular regions are not usually individually identifiable on CT. Each lentiform nucleus comprising the putamen (laterally) and the globus pallidus (medially) has, as its name implies, a lens shape with its apex directed medially (Fig. 2.1C). These structures are of slightly higher density than the surrounding white
matter. The third ventricle is a linear-shaped structure in the midline. The pineal gland and habenular calcifications are present in most adults at the posterior aspect of the third ventricle. Posterior and inferior to these calcifications is the quadrigeminal plate and its accompanying cistern (Fig. 2.1B). The quadrigeminal cistern has a W shape and continues laterally with the ambient cisterns and the perimesencephalic cisterns. The lateral ventricles are visualized in a piecemeal fashion. The frontal horns are indented laterally by the head of the caudate nuclei (Fig. 2.1C). The frontal horns have a narrow angle between them and are separated from each other by the septum pellucidum. Inferiorly, the temporal horns are visible in images that demonstrate the temporal lobes. The occipital horns are seen slightly superior and contiguous with the trigones (atria). The trigones are the largest part of the lateral ventricles and contain the commonly calcified glomi of the choroid plexi. The cephalad-most portion of the lateral ventricles are the bodies (Fig. 2.1D). The white matter tracts adjacent to the lateral ventricles consist of commissural fibers from the corpus callosum and projection fibers from the corona radiata. When the brain is sectioned horizontally, above the level of the lateral ventricles, deep white matter is seen as a semi-oval shaped area comprising intersecting bundles of commissural, projection, and association fibers known as the centrum semiovale. The interhemispheric fissure contains the falx cerebri, which may become calcified in adults.
matter. The third ventricle is a linear-shaped structure in the midline. The pineal gland and habenular calcifications are present in most adults at the posterior aspect of the third ventricle. Posterior and inferior to these calcifications is the quadrigeminal plate and its accompanying cistern (Fig. 2.1B). The quadrigeminal cistern has a W shape and continues laterally with the ambient cisterns and the perimesencephalic cisterns. The lateral ventricles are visualized in a piecemeal fashion. The frontal horns are indented laterally by the head of the caudate nuclei (Fig. 2.1C). The frontal horns have a narrow angle between them and are separated from each other by the septum pellucidum. Inferiorly, the temporal horns are visible in images that demonstrate the temporal lobes. The occipital horns are seen slightly superior and contiguous with the trigones (atria). The trigones are the largest part of the lateral ventricles and contain the commonly calcified glomi of the choroid plexi. The cephalad-most portion of the lateral ventricles are the bodies (Fig. 2.1D). The white matter tracts adjacent to the lateral ventricles consist of commissural fibers from the corpus callosum and projection fibers from the corona radiata. When the brain is sectioned horizontally, above the level of the lateral ventricles, deep white matter is seen as a semi-oval shaped area comprising intersecting bundles of commissural, projection, and association fibers known as the centrum semiovale. The interhemispheric fissure contains the falx cerebri, which may become calcified in adults.
TRAUMATIC BRAIN EMERGENCIES
Acute Hemorrhage
Computed Tomograpy
On CT, the normal gray matter has an attenuation value of 25 to 40 H units, whereas fresh blood in a patient with a normal hematocrit measures 40 to 70 units. This difference in density allows for recognition of fresh blood.
Magnetic Resonance Imaging
On magnetic resonance imaging (MRI), the signal characteristics of blood depend on the biochemical and oxidation characteristics of hemoglobin. The appearance of blood on MRI is extremely complex and also dependent on imaging parameters used. The following description applies only to intracerebral blood as imaged on 1.5T MR units. Freshly extravasated blood contains fully oxygenated hemoglobin (oxyhemoglobin) within intact red blood cells. This results in a weak diamagnetic effect, and the imaging appearance of a hyperacute hematoma is similar to that of CSF (isointense to gray matter in T1-weighted images [T1WIs] and hyperintense on T2-weighted images [T2WIs]). During the first few hours following a hemorrhage, oxyhemoglobin loses oxygen and becomes deoxyhemoglobin, which is still contained within intact erythrocytes. Deoxyhemoglobin has a diamagnetic effect and is of slightly low signal intensity on T1WI and low signal intensity on T2WI. This hypointensity is more pronounced on T2-weighted gradient recalled echo (T2*) sequences or susceptibility weighted images. The deoxyhemoglobin is oxidized into methemoglobin. Intracellular methemoglobin (3 to 7 days) is paramagnetic and is hyperintense on T1WI and hypointense on T2WI and T2*-weighted images. During the following days (> 5 days) and beginning at the periphery of the hematoma, erythrocytes lyse, liberating their contents. Extracellular methemoglobin is paramagnetic and is bright on T1- and T2-weighted sequences. Phagocytes ingest the methemoglobin and store it as ferritin. Once their capacity to store ferritin is reached, iron is stored as hemosiderin. Both of these compounds are paramagnetic and result in very low signal intensity on T1WI, T2WI, and T2*-weighted images. To further complicate this sequence of events, the signal characteristics of blood also depend on the state of hydration and retraction of the clot. The sequence of events described previously occurs in that order in the presence of uncomplicated hematomas. Deviations from this sequence may suggest the presence of an underlying lesion such as a tumor or a vascular malformation or rebleeding into a hematoma.
EXTRACEREBRAL TRAUMA
Epidural Hematoma
The potential epidural space is located between the outer layer of dura (periosteum) and the inner table of the skull. The dura is attached to the bone by Sharpe’s fibers and a forceful dissection is needed to strip it away and create a space. The dura is also firmly attached at the level of the cranial sutures. Epidural hematomas result from laceration of the
meningeal arteries and/or dural venous sinuses. The most common location (75%) for epidural hematomas is at the level of the middle meningeal artery and is caused by fractures involving the squamosal portion of the temporal bone. Approximately 95% of epidural hematomas have underlying fractures. The fractures per se, are of little clinical significance unless they are depressed. Most epidural hematomas are unilateral and supratentorial. Epidural hematomas have a better prognosis than subdural hematomas because the brain parenchyma is often less severely injured compared to patients with subdural hemorrhage.
meningeal arteries and/or dural venous sinuses. The most common location (75%) for epidural hematomas is at the level of the middle meningeal artery and is caused by fractures involving the squamosal portion of the temporal bone. Approximately 95% of epidural hematomas have underlying fractures. The fractures per se, are of little clinical significance unless they are depressed. Most epidural hematomas are unilateral and supratentorial. Epidural hematomas have a better prognosis than subdural hematomas because the brain parenchyma is often less severely injured compared to patients with subdural hemorrhage.
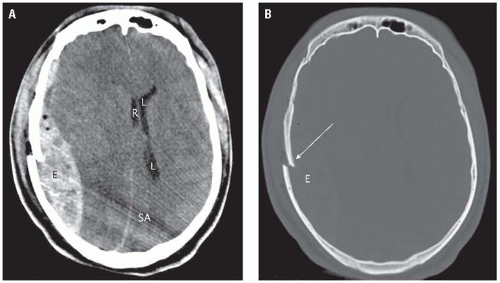
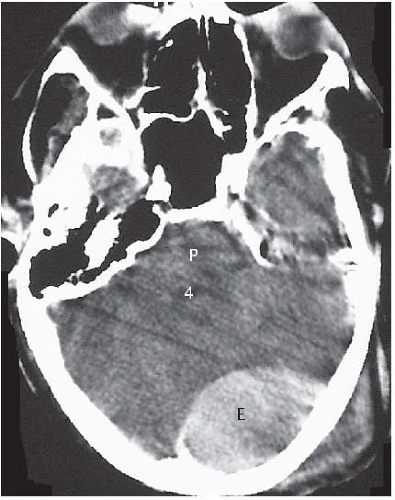
The imaging method of choice in head trauma patients is CT. Acute epidural hematomas have a density ranging from 50 to 70 units. Their shape is typically biconvex and their margins stop at the cranial sutures (Fig. 2.2A). Bone window settings allow for identification of underlying fractures (Fig. 2.2B). Because epidural hematomas are located outside of the dura, they may cross the midline and the venous sinuses. Epidural hematomas in the posterior fossa are more common in children than in adults. When an epidural hematoma is located nearby or above and below the tentorium, tear of the transverse sinus
should be strongly suspected (Fig. 2.3). Approximately 20% of patients with epidural hematomas also show blood in the subdural space. Eight percent of epidural hematomas are found in follow-up imaging studies and do not present acutely (delayed epidural hematomas).
should be strongly suspected (Fig. 2.3). Approximately 20% of patients with epidural hematomas also show blood in the subdural space. Eight percent of epidural hematomas are found in follow-up imaging studies and do not present acutely (delayed epidural hematomas).
Subdural Hematoma
These collections are located between the inner layer of the dura and the arachnoid membrane. Because this space is easily dissected, subdural hematomas tend to be extensive. They arise from injury to the bridging veins (crossing between the arachnoid membrane and the dura) and most are located primarily in the frontoparietal regions. Approximately 10% to 15% of subdural hematomas are bilateral. Brain contusions, hemorrhages, and shearing injuries are present in 50% of patients with subdural hematomas. The mortality because of subdural hematomas with concomitant brain injuries is high (60% to 90%). The mortality for isolated subdural hematomas is lower (20%). Bilateral convexity or interhemispheric subdural hematomas in a child should raise the suspicion of non-accidental trauma (see NAHI: Child Abuse, subsequently).
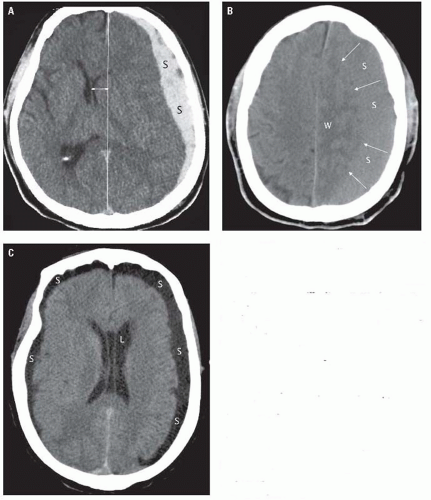
Acute subdural hematomas (< 7 days) are usually hyperdense and measure 50 to 70 units (Fig. 2.4A). Subdural hematomas have a crescentic shape. They
are generally accompanied by significant mass effect. Between 1 and 3 weeks, subdural hematomas become isodense with respect to brain and may be difficult to visualize. In these patients, recognizing the displacement of the gray-white junction away from the inner table of the skull may facilitate the diagnosis (Fig. 2.4B). Chronic subdural hematomas (> 3 weeks) are hypodense with respect to brain (Fig. 2.4C). Identification of small subdural hematomas can be aided by the use of intermediate (blood) window settings (width: 250 and level: 40) (Fig. 2.5A). Subdural hematomas may follow the dural reflections and be seen along the falx anteriorly or posteriorly (Fig. 2.5A), the tentorium (Fig. 2.5B), or in the interhemispheric fissure superiorly (Fig. 2.5C). Repeated episodes of bleeding into a subdural hematoma may result in a layered or swirled appearance. Delayed subdural hematomas are less common than delayed epidural hematomas. Although MRI does not play a role in the initial diagnosis of these lesions, it is extremely helpful in the identification of subacute and chronic subdural
hematomas. MRI is particularly helpful when attempting to establish the presence of subdural hematomas of varying ages. In cases of inflicted head trauma in child abuse, MRI is essential for assessing subacute and chronic injury.
are generally accompanied by significant mass effect. Between 1 and 3 weeks, subdural hematomas become isodense with respect to brain and may be difficult to visualize. In these patients, recognizing the displacement of the gray-white junction away from the inner table of the skull may facilitate the diagnosis (Fig. 2.4B). Chronic subdural hematomas (> 3 weeks) are hypodense with respect to brain (Fig. 2.4C). Identification of small subdural hematomas can be aided by the use of intermediate (blood) window settings (width: 250 and level: 40) (Fig. 2.5A). Subdural hematomas may follow the dural reflections and be seen along the falx anteriorly or posteriorly (Fig. 2.5A), the tentorium (Fig. 2.5B), or in the interhemispheric fissure superiorly (Fig. 2.5C). Repeated episodes of bleeding into a subdural hematoma may result in a layered or swirled appearance. Delayed subdural hematomas are less common than delayed epidural hematomas. Although MRI does not play a role in the initial diagnosis of these lesions, it is extremely helpful in the identification of subacute and chronic subdural
hematomas. MRI is particularly helpful when attempting to establish the presence of subdural hematomas of varying ages. In cases of inflicted head trauma in child abuse, MRI is essential for assessing subacute and chronic injury.
Subdural Hygroma
A collection of CSF in the subdural space is termed a hygroma. They are thought to result from laceration of the arachnoid membrane and extravasation of nonbloody CSF into the subdural space. The incidence of subdural hygroma is not known, but they are less common than subdural hematomas. Subdural hygromas have a shape similar to subdural hematomas, but their CT density is equal to that of CSF (Fig. 2.6). By CT, subdural hygromas may be indistinguishable from chronic subdural hematomas or widened subarachnoid spaces in the elderly or other patients with significant brain atrophy. Small subdural hygromas do not require evacuation and resolve spontaneously within several months.
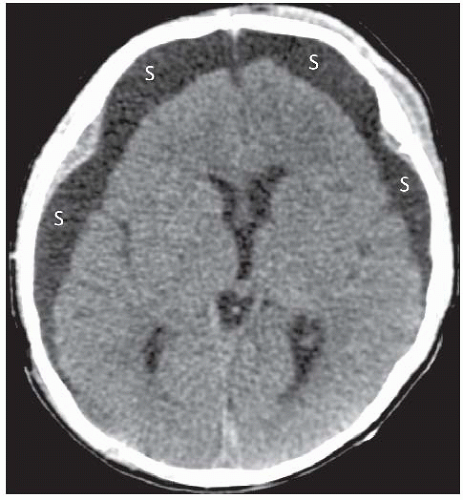 Figure 2.6. Acute subdural hygroma
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|