Cysts
The simple cyst is a fluid-filled cavity with a lining consisting of an inner epithelial layer and an outer myoepithelial layer.
Characteristics
Incidence: very common.
Peak age: all age groups; incidence decreases after menopause.
Multifocality: frequent.
Bilateral occurrence: frequent.
Complications: none.
Risk of malignancy: less than 1%.
Prognosis: excellent.
Histological classification after biopsy: B2.
Implications for work-up/treatment. None if symptom-free. If there is pain, ultrasound-guided cyst aspiration, preferably by fine needle, may be performed.
Shape Round, oval.
Margins Circumscribed.
Internal composition Homogeneous fluid
Clinical findings Small cysts normally have no correlative clinical findings. In the case of larger cysts, a circumscribed, firm-elastic mass may be palpable. Occasionally there is tenderness.
Imaging
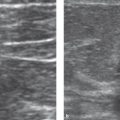
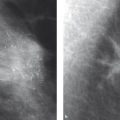
Sonography (▶ Fig. 8.1a): Anechoic mass; posterior acoustic enhancement; unambiguous sonographic finding.
Mammography (▶ Fig. 8.1b): mass with parenchyma-equivalent, homogeneous density; difficult or impossible to detect within dense breast tissue structures; good visibility within the lipomatous breast; occasional cyst wall calcifications.
Breast MRI (▶ Fig. 8.1c): mass:
T1W precontrast image: hypointense.
T2W image: hyperintense.
After administration of contrast medium: no enhancement.
Note
Mammography cannot reliably distinguish between a cyst (fluid-filled) and a proliferative process (solid tissue).
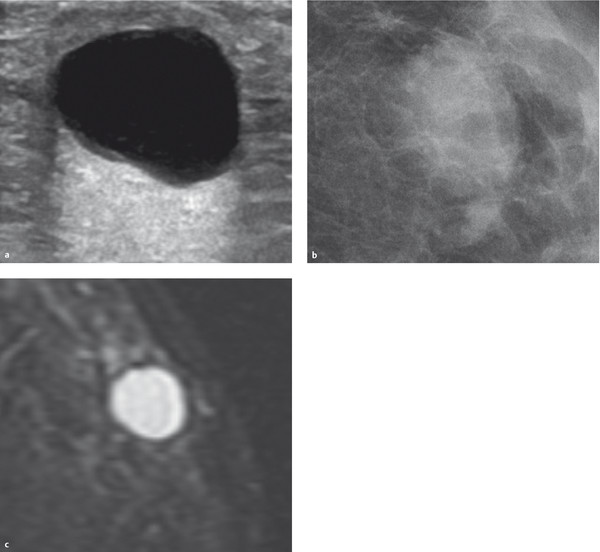
Fig. 8.1 Simple cyst. (a) Ultrasound image. (b) Mammogram. (c) T2W breast MR image.
Differential diagnosis Inflamed cyst, complex cyst, cyst with intracystic proliferation, triple-negative carcinoma.
8.1.2 Inflamed Cysts
The inflamed cyst is a simple cyst with inflamed—and thus hyperemic—cyst walls.
Characteristics
Incidence: rare.
Peak age: all age groups; incidence decreases after menopause.
Multifocality: rare.
Bilateral occurrence: rare.
Complications: occasional pain.
Risk of malignancy: less than 1%.
Prognosis: excellent.
Histological classification after biopsy: B2.
Implications for work-up/treatment None if symptom-free. If there is pain, ultrasound-guided cyst aspiration, preferably by fine needle, may be performed.
Shape Round, oval.
Margins Predominantly circumscribed, occasionally slightly indistinct.
Internal composition Homogeneous fluid.
Clinical findings Small cysts normally have no correlative clinical findings. In the case of larger cysts, a circumscribed, firm-elastic, occasionally tender mass may be palpable.
Imaging
Sonography (▶ Fig. 8.2a): anechoic mass, cyst wall mildly thickened, possibly indistinct; posterior acoustic enhancement.
Mammography (▶ Fig. 8.2b): mass with parenchyma-equivalent, homogeneous density; difficult or impossible to detect within dense breast tissue structures; good visibility within the lipomatous breast; occasional cyst wall calcifications.
Breast MRI (▶ Fig. 8.2c): mass:
T1W precontrast image: hypointense.
T2W image: hyperintense.
After administration of contrast medium: cyst wall enhancement.
Note
When interpreting breast MRI, one should use the term “cyst wall enhancement” rather than “rim enhancement,” because the term “rim enhancement” is closely associated with malignancy.

Fig. 8.2 Inflamed cyst. (a) Ultrasound image. (b) Mammogram. (c) Contrast-enhanced breast MRI subtraction image.
Differential diagnosis Complex cyst, cyst with intracystic proliferation, triple-negative carcinoma.
8.1.3 Complex Cysts
When bleeding occurs into a simple cyst, it is referred to as a complex cyst (synonyms: hemorrhagic cyst, chocolate cyst). A cyst filled with highly proteinaceous fluid also justifies use of the term “complex cyst.”
Characteristics
Incidence: rare.
Peak age: all age groups; incidence decreases after menopause.
Multifocality: rare.
Bilateral occurrence: rare.
Complications: none.
Risk of malignancy: less than 1%.
Prognosis: excellent.
Histological classification after biopsy: B2.
Implications for work-up/treatment None if symptom-free. If there is pain, ultrasound-guided cyst aspiration using a larger gauge needle (10G) may be indicated.
Shape Round, oval.
Margins Circumscribed.
Internal composition Homogeneous to inhomogeneous due to fresh blood (with sedimentation effect), old blood, protein.
Clinical findings Small lesions normally have no correlative clinical findings. In the case of larger cysts, a circumscribed, firm-elastic mass may be palpable. The lesions are rarely tender to palpation.
Imaging
Sonography (▶ Fig. 8.3a): hypoechoic mass; minor posterior acoustic enhancement (protein), moderate acoustic enhancement to acoustic extinction (old blood).
Mammography (▶ Fig. 8.3b): mass with parenchyma-equivalent, homogeneous density; difficult or impossible to detect within dense breast tissue structures; good visibility within the lipomatous breast.
Breast MRI (▶ Fig. 8.3c): mass:
T1W precontrast image: isointense (protein) to hypointense (old blood); sedimentation may be visible.
T2W image: isointense (protein) to hypointense (old blood); sedimentation may be visible.
After administration of contrast medium: no to slight cyst wall enhancement.
Note
When there is uncertainty in the differential diagnosis of a complex breast cyst, percutaneous biopsy should be undertaken.

Fig. 8.3 Hemorrhagic cyst. (a) Ultrasound image. (b) Mammogram. (c) T2W breast MR image.
Differential diagnosis Inflamed cyst, cyst with intracystic proliferation, triple-negative carcinoma, carcinoma with central degeneration.
8.1.4 Myxoid Fibroadenoma
Fibroadenomas are benign, mixed fibroepithelial tumors. In younger women, they usually present with a high epithelial content (myxoid fibroadenoma).
Characteristics
Incidence: frequent.
Peak age: 18 to 50 years.
Multifocality: frequent.
Bilateral occurrence: frequent.
Complications: usually none. The juvenile giant fibroadenoma is an exception due to its massive size (diameter up to 12–15 cm).
Risk of malignancy: 0%.
Prognosis: excellent.
Histological classification after biopsy: B2.
Implications for work-up/treatment For smaller lesions in younger women (up to 30 years of age), no treatment is required. For smaller lesions in women aged 30 years and above, diagnosis should be histologically confirmed via percutaneous, preferably ultrasound-guided, core needle biopsy. Giant fibroadenomas should be surgically excised.
Shape Lobular, oval.
Margins Circumscribed.
Internal composition Homogeneous, proliferative tissue.
Clinical findings Small cysts normally have no correlative clinical findings. In the case of larger myxoid fibroadenomas, circumscribed lumps may be palpable.
Imaging
Sonography (▶ Fig. 8.4a): homogeneous, isoechoic mass; longitudinal axis parallel to the skin; mild elasticity; mild posterior acoustic enhancement.
Mammography (▶ Fig. 8.4b): mass with parenchyma-equivalent, homogeneous density; difficult or impossible to detect within dense breast tissue structures; good visibility within the lipomatous breast; rarely displays endotumoral microcalcifications.
Breast MRI (▶ Fig. 8.4c): focus, mass:
T1W precontrast image: hypointense.
T2W image: hyperintense; may display endotumoral hypointense septations with increasing fibrosis.
After administration of contrast medium: strong homogeneous enhancement; may display nonenhancing endotumoral septations with increasing fibrosis (dark septations).
Note
Myxoid fibroadenomas are the most common tumors in young women.

Fig. 8.4 Myxoid fibroadenoma. Fibroadenoma with high water content. (a) Ultrasound image. (b) Mammogram. (c) Contrast-enhanced breast MRI subtraction image.
Differential diagnosis Adenoma, papilloma, phyllodes tumor, invasive carcinoma, intramammary metastasis.
8.1.5 Fibrotic Fibroadenoma
Fibroadenomas are benign, mixed fibroepithelial tumors. With increasing age, the fibrous component increasingly predominates (fibrotic fibroadenoma; synonyms: hyaline fibroadenoma, regressive fibroadenoma).
Characteristics
Incidence: frequent.
Peak age: 40 to 80 years.
Multifocality: frequent.
Bilateral occurrence: frequent.
Complications: none.
Risk of malignancy: 0%.
Prognosis: excellent.
Histological classification after biopsy: B2.
Implications for work-up/treatment When there is uncertainty in the differential diagnosis, percutaneous, preferably ultrasound-guided, core needle biopsy should be undertaken.
Shape Lobular, oval.
Margins Circumscribed.
Internal composition Inhomogeneous to homogeneous proliferative tissue.
Clinical findings Small lesions normally have no correlative clinical findings. In the case of a larger fibrotic fibroadenoma, a circumscribed, rather firm mass may be palpable.
Imaging
Sonography (▶ Fig. 8.5a): inhomogeneous or homogeneous, hypoechoic to isoechoic mass; longitudinal axis parallel to the skin; no elasticity; intermediate posterior echo pattern to acoustic extinction (acoustic extinction is particularly seen in the case of endotumoral macrocalcifications).
Mammography (▶ Fig. 8.5b): mass with parenchyma-equivalent, homogeneous density; difficult or impossible to detect within dense breast tissue structures; good visibility within the lipomatous breast; frequently displays endotumoral macrocalcifications (popcornlike).
Breast MRI (▶ Fig. 8.5c): focus, mass:
T1W precontrast image: isointense; endotumoral signal loss in the case of macrocalcifications.
T2W image: isointense; endotumoral signal loss in the case of macrocalcifications.
After administration of contrast medium: minor or no enhancement.
Note
Popcornlike endotumoral macrocalcifications on mammography are pathognomonic for a fibrotic fibroadenoma.

Fig. 8.5 Fibrous fibroadenoma. Fibroadenoma with regressive changes. (a) Ultrasound image. (b) Mammogram. (c) T1W precontrast breast MR image.
Differential diagnosis Adenoma, papilloma, phyllodes tumor, invasive carcinoma, intramammary metastasis.
8.1.6 Adenoma
The adenoma, as opposed to the fibroadenoma, exhibits only a very minor stromal component. It is a circumscribed tumor with tubular structures and a surrounding pseudocapsule.
Characteristics
Incidence: rare.
Peak age: usually in younger women up to 50 years of age.
Multifocality: rare.
Bilateral occurrence: rare.
Complications: none.
Risk of malignancy: 0%.
Prognosis: excellent.
Histological classification after biopsy: B2.
Implications for work-up/treatment For smaller lesions in younger women (up to 30 years of age), no treatment is required. For smaller lesions in women aged 30 years and above, confirmation of the diagnosis via percutaneous, preferably ultrasound-guided, core needle biopsy should be undertaken.
Shape Round, oval, lobular.
Margins Circumscribed.
Internal composition Usually homogeneous proliferative tissue; may rarely display lipomatous inclusions.
Clinical findings Small lesions normally have no correlative clinical findings. In the case of a larger adenoma, a circumscribed, rather soft mass may be palpable.
Imaging
Sonography (▶ Fig. 8.6a): homogeneous, isoechoic mass; longitudinal axis parallel to the skin; good elasticity; mild posterior acoustic enhancement.
Mammography (▶ Fig. 8.6b): mass with parenchyma-equivalent, homogeneous density; difficult or impossible to detect within dense breast tissue structures; good visibility within the lipomatous breast; may rarely display endotumoral microcalcifications.
Breast MRI (▶ Fig. 8.6c): focus, mass:
T1W precontrast image: hypointense.
T2W image: hyperintense.
After administration of contrast medium: moderate to strong homogeneous enhancement.
Note
Adenoma of the nipple represents a variant form of adenoma. It grows in the terminal portion of the nipple and is preferably treated with surgical excision (differential diagnosis: eczema, Paget’s disease).

Fig. 8.6 Adenoma. (a) Ultrasound image. (b) Mammogram. (c) Contrast-enhanced breast MRI subtraction image.
Differential diagnosis Fibroadenoma, papilloma, phyllodes tumor, invasive carcinoma.
8.1.7 Hamartoma
The hamartoma (synonym: fibroadenolipoma, lipofibroadenoma, “breast within the breast”) is a circumscribed mixed tumor with organoid composition and pseudocapsular margins.
Characteristics
Incidence: rare.
Peak age: all age groups.
Multifocality: rare.
Bilateral occurrence: rare.
Complications: none.
Risk of malignancy: 0%.
Prognosis: excellent.
Histological classification after biopsy: B2.
Implications for work-up/treatment: None. When there is uncertainty in the differential diagnosis, percutaneous, preferably ultrasound-guided, core needle biopsy, should be undertaken and should include portions of the pseudocapsule.
Shape Round, oval, lobular.
Margins Circumscribed; the lesion displays a pseudocapsule.
Internal composition Inhomogeneous parenchyma-equivalent tissue (fat, parenchyma, vessels).
Clinical findings Small lesions normally have no correlative clinical findings. In the case of a larger hamartoma, a circumscribed mass may be palpable. The consistency is dependent upon the composition.
Imaging
Sonography (▶ Fig. 8.7a): inhomogeneous mass with echogenicity depending upon the composition (fat and parenchymal components).
Mammography (▶ Fig. 8.7b): mass with hyperdense to hypodense tumor areas depending upon the endotumoral composition; impression of a “breast within the breast” (termed so by the pathologist Thomas Bässler); very rarely displays endotumoral microcalcifications.
Breast MRI (▶ Fig. 8.7c): mass:
T1W precontrast image: mixed presentation (hypointense to hyperintense), depending upon composition.
T2W image: mixed presentation (hypointense to hyperintense), depending upon composition.
After administration of contrast medium: usually displays inhomogeneous enhancement of the parenchymal tumor areas.
Note
The composition and perfusion pattern of a hamartoma can be differentiated distinctly from the characteristics of the normal glandular tissue.

Fig. 8.7 Hamartoma. Mixed tumor. (a) Ultrasound image. (b) Mammogram. (c) T1W precontrast breast MR image.
Differential diagnosis Phyllodes tumor.
8.1.8 Lipoma
The lipoma (synonym: adipose tumor) is an encapsulated tumor that contains exclusively mature adipose cells.
Characteristics
Incidence: rare.
Peak age: all age groups.
Multifocality: rare.
Bilateral occurrence: rare.
Complications: none.
Risk of malignancy: 0%.
Prognosis: excellent.
Histological classification after biopsy: B2.
Implications for work-up/treatment None.
Shape Oval.
Margins Circumscribed, displays a delicate capsule.
Internal composition Homogeneous fat.
Clinical findings Small lesions normally have no correlative clinical findings. In the case of a larger lipoma, a circumscribed, soft, cushionlike mass may be palpable.
Imaging
Sonography (▶ Fig. 8.8a): hyperechoic mass (due to the arrangement of the adipose cells, echogenicity is frequently higher than that of subcutaneous or intramammary adipose tissue).
Mammography (▶ Fig. 8.8b): hypodense (fat-equivalent) mass with delicate surrounding capsule; no associated microcalcifications.
Breast MRI (▶ Fig. 8.8c): mass:
T1W precontrast image: hyperintense.
T2W image: hyperintense; hypointense in T2W fat-suppressed.
After administration of contrast medium: no enhancement.
Note
Liposarcoma does not develop from a breast lipoma.

Fig. 8.8 Lipoma. Adipose tumor. (a) Ultrasound image. (b) Mammogram. (c) T1W precontrast breast MR image.
Differential diagnosis Free intramammary adipose tissue.
8.1.9 Mammary Fibrosis
Mammary fibrosis is a regional or diffuse proliferation of the stroma with obliteration of the lactiferous ducts.
Characteristics
Incidence: frequent.
Peak age: all age groups.
Multifocality: frequent.
Bilateral occurrence: frequent.
Complications: none.
Risk of malignancy: not increased.
Prognosis: excellent.
Histological classification after biopsy: B2.
Implications for work-up/treatment None. When there is uncertainty in the differential diagnosis, percutaneous biopsy should be undertaken.
Shape, distribution Irregular, regional.
Margins Indistinct.
Internal composition Inhomogeneous proliferative tissue.
Clinical findings Small lesions normally have no correlative clinical findings. An areas of extensive fibrosis may be palpable as a firm mass.
Imaging
Sonography (▶ Fig. 8.9a): nonspecific inhomogeneous, hypoechogenic area, frequently occult.
Mammography (▶ Fig. 8.9b): inhomogeneous, hyperdense area, occasionally presenting as a focal asymmetry or density; nonspecific; rarely associated with microcalcifications.
Breast MRI (▶ Fig. 8.9c): non-space-occupying lesion (nonmasslike lesion):
T1W precontrast image: intermediary signal, nonspecific.
T2W image: intermediary signal, nonspecific.
After administration of contrast medium: mild to strong enhancement, nonspecific.
Note
Mammary fibrosis is usually visualized as ambiguous microcalcifications on the mammogram or as a nonmasslike area of increased enhancement in the MRI.

Fig. 8.9 Mammary fibrosis. (a) Ultrasound image. (b) Mammogram. (c) Contrast-enhanced breast MRI subtraction image.
Differential diagnosis Adenosis, DCIS, ILC.
8.1.10 Adenosis of the Breast
Adenosis (synonym: adenosis mammae) is a clustered proliferation of the small ductal segments and terminal ducts. Histopathology differentiates the following types: sclerosing adenosis, microcystic adenosis (blunt-duct adenosis), and the less common microglandular adenosis and the radial scar (see ▶ 8.2.2).
Characteristics
Incidence: frequent.
Peak age: all age groups.
Multifocality: frequent.
Bilateral occurrence: frequent.
Complications: none.
Risk of malignancy: not increased.
Prognosis: excellent.
Histological classification after biopsy: B2.
Implications for work-up/treatment None. When there is uncertainty in the differential diagnosis, percutaneous biopsy should be undertaken.
Shape, distribution Irregular; regional or multiregional.
Margins Indistinct.
Internal composition Inhomogeneous proliferative tissue.
Clinical findings Small lesions normally have no correlative clinical findings. A larger area of adenosis may be palpable as an area of increased firmness.
Imaging
Sonography (▶ Fig. 8.10a): nonspecific, inhomogeneous, hypoechogenic area, frequently occult.
Mammography (▶ Fig. 8.10b): inhomogeneous, hyperdense area, occasionally presenting as a focal asymmetry or density; nonspecific; frequently associated with microcalcifications (monomorphic, round); in the case of blunt-duct adenosis, often associated with amorphous calcifications in lobular distribution.
Breast MRI (▶ Fig. 8.10c): non-space-occupying lesion (nonmasslike lesion):
T1W precontrast image: intermediary signal, nonspecific.
T2W image: intermediary signal, nonspecific.
After administration of contrast medium: often displays strong enhancement, nonspecific.
Note
Circumscribed adenosis is the most common cause of false-positive findings on breast MRI because it exhibits an irregular shape and is often associated with increased perfusion.

Fig. 8.10 Adenosis. Sclerosing adenosis. (a) Ultrasound image. (b) Mammogram. (c) Nodular adenosis in the T1W precontrast breast MR image.
Differential diagnosis Mammary fibrosis, DCIS, ILC.
8.1.11 Fibrocystic Condition of the Breast
Morphological components of the fibrocystic condition of the breast (also referred to as fibrocystic mastopathy, fibrocystic breasts, fibrocystic disease of the breast, fibrocystic changes) are: micro- and macrocysts in varying degrees; hyperplasia of the lobules; epithelial hyperplasia of the lactiferous ducts and acini; and stromal fibrosis.
Characteristics
Incidence: frequent.
Peak age: all age groups.
Multifocality: frequent.
Bilateral occurrence: obligatory.
Complications: none.
Risk of malignancy: not increased.
Prognosis: excellent.
Histological classification after biopsy: B1.
Implications for work-up/treatment None. When there is uncertainty in the differential diagnosis, percutaneous biopsy should be undertaken.
Shape, distribution Irregular, diffuse, rarely regional.
Margins Indistinct.
Internal composition Inhomogeneous proliferative tissue + cysts.
Clinical findings Unremarkable to mild or moderately increased firmness.
Imaging
Sonography (▶ Fig. 8.11a): Generally hyperechogenic breast tissue; hypoechoic tubular changes (ectatic milk ducts); anechoic lesions with posterior acoustic enhancement (cysts); often bilaterally symmetric.
Mammography (▶ Fig. 8.11b): circumscribed or diffuse areas of hyperdensity; frequently associated with micro- and macrocalcifications; often bilaterally symmetric.
Breast MRI (▶ Fig. 8.10c): non-space-occupying lesion (nonmasslike lesion):
T1W precontrast image: hypointense lesions (cysts); otherwise nonspecific.
T2W image: hyperintense lesions (cysts); otherwise nonspecific.
After administration of contrast medium: no/slight/strong enhancement, sparing the cystic lesions; nonspecific.
Note
In general, the term, “mastopathy,” should be avoided and regarded as archaic. Currently it is only used in association with fibrocystic changes.

Fig. 8.11 Fibrocystic condition of the breast. (a) Ultrasound image. (b) Mammogram. (c) T2W breast MR image.
Differential diagnosis Fibrosis, adenosis, any diffuse tumor manifestation.
8.1.12 Adenomyoepithelioma
An adenomyoepithelioma is a circumscribed nodule consisting of a proliferation of myoepithelial cells.
Characteristics
Incidence: very rare.
Peak age: all age groups.
Multifocality: very rare.
Bilateral occurrence: very rare.
Complications: none.
Risk of malignancy: 0%.
Prognosis: excellent.
Histological classification after biopsy: B2.
Implications for work-up/treatment None. When there is uncertainty in the differential diagnosis, percutaneous biopsy should be undertaken.
Shape Round, oval.
Margins Circumscribed or indistinct.
Internal composition Inhomogeneous proliferative tissue.
Clinical findings Small lesions normally have no correlative clinical findings. Larger lesions may present as circumscribed, usually firm nodules.
Imaging
Sonography (▶ Fig. 8.12a): hypoechogenic mass, nonspecific.
Mammography (▶ Fig. 8.12b): hyperdense mass, nonspecific.
Breast MRI (▶ Fig. 8.12c): focus, mass:
T1W precontrast image: parenchyma-equivalent, nonspecific.
T2W image: parenchyma-equivalent, nonspecific.
After administration of contrast medium: moderate to strong enhancement; may display postinitial washout.
Note
Adenomyoepitheliomas do not exhibit any specific imaging characteristics. Due to their increased vascularity, they are frequently false-positive findings in MRI.

Fig. 8.12 Adenoepithelioma. (a) Ultrasound image. (b) Mammogram. (c) Contrast-enhanced breast MRI subtraction image.
Differential diagnosis Fibroadenoma, papilloma, phyllodes tumor, carcinoma of the breast.
8.1.13 Acute Nonpuerperal Mastitis
Acute nonpuerperal mastitis of the breast (synonym: acute inflammation of the breast) is a predominantly interstitial, but occasionally also intraductal, inflammation of the parenchyma outside the lactation period.
Characteristics
Incidence: rare.
Peak age: all age groups.
Multifocality: may extend over the entire breast.
Bilateral occurrence: very rare.
Complications: abscess formation; development of fistulas.
Risk of malignancy: 0%.
Prognosis: good.
Histological classification after biopsy: B2.
Implications for work-up/treatment Primary treatment of the inflammation: immobilization, cooling, administration of antibiotics. If the symptoms do not completely regress in 7 to 10 days, confirm the diagnosis with a percutaneous biopsy or open representative excision.
Shape, distribution Irregular, frequently segmental or diffuse (entire breast).
Margins Indistinct.
Internal composition Inhomogeneous proliferative, edematous tissue.
Clinical findings Inflammation triad: warmth to the touch, redness, pain. Possible swelling, skin thickening, lymphadenitis, and fever. May be associated with increased laboratory inflammatory markers.
Imaging
Sonography (▶ Fig. 8.13a): Hyperechogenicity of the parenchyma and subcutaneous space (edematous imbibition-compare with the contralateral breast), skin thickening, locoregional lymphadenitis.
Mammography (▶ Fig. 8.13b): Hyperdensity and decreased transparency (tissue structures with a “washed-out appearance”—compare with the contralateral breast!), skin thickening, locoregional lymphadenitis.
Breast MRI (▶ Fig. 8.13c): non-space-occupying lesion (nonmasslike lesion):
T1W precontrast image: no specific intramammary changes; skin thickening; locoregional lymphadenitis.
T2W image: hyperintensity of the affected parenchymal areas; skin thickening; locoregional lymphadenitis.
After administration of contrast medium: increased enhancement of the thickened skin; often greatly increased perfusion of the inflamed parenchymal areas.
Note
It is frequently difficult or impossible to distinguish between nonpuerperal mastitis and inflammatory breast cancer on a clinical or imaging basis.

Fig. 8.13 Acute nonpuerperal mastitis. (a) Ultrasound image. (b) Mammogram. (c) Contrast-enhanced breast MRI subtraction image.
Differential diagnosis Inflammatory breast cancer.
8.1.14 Chronic Nonpuerperal Mastitis
Chronic nonpuerperal mastitis (synonym: chronic abacterial inflammation of the breast) is a chronic, abacterial, inflammatory process that occurs in and around the terminal ducts and lobules of the breast. Histopathology differentiates the following 3 forms: galactophoritis, granulomatous mastitis, and lobular mastitis. Plasma cell mastitis is the final stage of circumductal mastitis.
Characteristics
Incidence: rare.
Peak age: higher age groups.
Multifocality: very rare.
Bilateral occurrence: very rare.
Complications: none.
Risk of malignancy: 0%.
Prognosis: excellent.
Histological classification after biopsy: B2.
Implications for work-up/treatment None. When there is uncertainty in the differential diagnosis, percutaneous biopsy should be undertaken.
Shape, distribution Irregular, frequently ductal or segmental.
Margins Indistinct.
Internal composition Inhomogeneous proliferative tissue.
Clinical findings Small lesions normally have no correlative clinical findings. Rarely associated with pain, nipple discharge, or retraction of the nipple.
Imaging
Sonography (▶ Fig. 8.14a): hypoechogenic tissue; hypoechoic tubular changes (mammary duct ectasia); nonspecific.
Mammography (▶ Fig. 8.14b): hyperdensity; trabecular thickening; retroareolar ductal ectasia; in its final stage radially arranged, lancetlike calcifications are classic (plasma cell mastitis).
Breast MRI (▶ Fig. 8.14c): non-space-occupying lesion (nonmasslike lesion):
T1W precontrast image: hypointense, nonspecific.
T2W image: hyperintense, nonspecific; occasionally ectatic milk ducts.
After administration of contrast medium: mild to strong enhancement, regional or segmental.
Note
The chronic inflammation associated with chronic nonpuerperal mastitis is not typically associated with any symptoms.

Fig. 8.14 Chronic mastitis. (a) Ultrasound image of chronic-segmental inflammation. (b) Plasma cell mastitis on mammography. (c) Chronic, segmental inflammation in the contrast-enhanced breast MRI subtraction image.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree