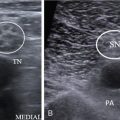
Reetu Amrita John, Anuradha Chandramohan, Aliva A. Nayak, Saumya Sara Sunny Primary hyperparathyroidism is the third most common endocrine disorder after diabetes and thyroid disease and occurs due to excessive production of parathyroid hormone. The overall incidence of primary hyperparathyroidism is 1–2 per 1000 with higher incidence among those over 50 years, and it is two to three times more common among women. The majority (90%) of primary hyperparathyroidism is sporadic and rest being familial (10%). Familial syndromes associated with primary hyperparathyroidism include multiple endocrine neoplasia syndrome (MEN-1 and MEN-2A) and isolated familial hyperparathyroidism associated with jaw tumour syndrome. The most common cause of primary hyperparathyroidism is due to a single parathyroid adenoma (80%–85%) and less commonly due to two or more adenomas (4%–5%). Parathyroid hyperplasia (10%–15%) and very rarely parathyroid carcinoma (less than 1%) also cause primary hyperparathyroidism. Secondary hyperparathyroidism is associated with chronic renal disease. Chronic renal insufficiency may lead to autonomous PTH secretion due to chronic stimulation of the parathyroid glands, and this phenomenon is called tertiary hyperparathyroidism. Surgical removal of the diseased parathyroid gland or glands remains the main stay of treatment of primary hyperparathyroidism with a very high cure rate of more than 95% in high volume centres. Accurate preoperative localization of the parathyroid lesion is the key to high surgical cure rates with least morbidity. This is most commonly achieved with a combination of anatomical and functional imaging modalities. Ultrasound neck and nuclear scintigraphy are the first-line imaging performed for localization of abnormal parathyroid. Four-dimensional computed tomography (4D-CT), MRI and selective venous sampling are used as a problem-solving tool in a very small number of patients. In this chapter, we review the role of different imaging modalities in the management of hyperparathyroidism. The parathyroid glands start to develop at sixth week of foetal life and migrate caudally at eighth week. The superior parathyroid glands arise from the fourth branchial pouch and are in close association with the lateral lobes of the thyroid. There is only minimal descent of these glands, and hence, their position is relatively constant with only 2% being ectopic. The inferior parathyroid glands arise from the third branchial pouch along with the thymus and hence have a longer embryologic descent than the superior pair, explaining their variable anatomic positions including the intrathymic location. Superior parathyroid glands are deep to the plane of the recurrent laryngeal nerve (RLN). They are most commonly posterior to the middle one-third of the lateral lobes of the thyroid gland (75%). Inferior parathyroid glands are superficial to the plane of the RLN and the most common anatomic location being posterior and lateral to the lower pole of the thyroid gland (50%) and the next most common site being 1 cm below the lower pole of thyroid. Ectopy of one or more parathyroid glands is seen in 5%–15%, with the inferior parathyroid glands more commonly ectopic than the superior. Table 3.36.1 shows a list of ectopic locations of the parathyroid gland. While the majority have four parathyroid glands, supernumerary glands up to 12 in number are seen in 2.5%–22% and less than four parathyroid glands are seen in 3%. Parathormone regulates calcium homeostasis by acting on the bones, gastrointestinal tract and the kidneys. In the bones, there is increased osteoclastic bone resorption and removal of the calcium phosphate salts causing mobilization of calcium from the bone into the plasma. In the kidneys, there is increased tubular reabsorption of calcium along with loss of phosphate. In the gastrointestinal tract, it increases production of Vitamin D, which causes increased calcium absorption. Thus, patients with primary hyperparathyroidism have increased levels of serum PTH and serum calcium levels. Other tests that can be done include 24-h urinary calcium and vitamin D levels. Many patients are diagnosed on routine screening and hence may be asymptomatic or show nonspecific symptoms such as fatigue, depression, muscle pains, decreased concentration and memory loss. The more specific clinical manifestations include nephrolithiasis and nephrocalcinosis, pancreatitis, osteopaenia, neuropsychiatric manifestations, cardiovascular morbidity and rarely hypercalcaemic crisis. Multiple endocrine neoplasia occurs due to a genetic defect that results in two or more endocrine related tumours. The two main forms are MEN1 and MEN-2, each of which have tumours from specific organs. MEN-1 syndrome is characterized by parathyroid, pancreatic and pituitary tumours as the major components. Primary hyperparathyroidism is the most common presentation, seen in more than 90% of patients, and there is typically multiglandular involvement. MEN 2A syndrome is associated with medullary carcinoma of thyroid, pheochromocytomas and parathyroid lesions. In this syndrome, hyperparathyroidism is seen in 20%–30% and is due to parathyroid hyperplasia, adenoma or both entities together. MEN-1 syndrome is associated with parathyroid hyperplasia and supernumerary parathyroid glands. MEN 2A syndrome is associated with parathyroid adenoma. Hyperparathyroidism with jaw tumour syndrome (HPT-JT) may have single or multiglandular disease, and 15% of them may have parathyroid carcinoma. Parathyroid carcinoma is a rare cause of hyperparathyroidism and these patients have severe symptoms of hyperparathyroidism at presentation, very high serum PTH levels and clinically palpable hard neck mass. Ultrasound is the initial imaging modality for assessment of parathyroid adenomas. It is performed using a high-resolution linear array transducer (7.5–10 MHz). The patient is in supine position with the neck mildly hyperextended. Examination should be done in the longitudinal and transverse planes. The region of scanning should include both perithyroidal areas and the carotid sheaths and must extend from the angle of the mandible superiorly to the sternal notch inferiorly. Normal-sized parathyroid glands are usually not identified by most imaging modalities due to their small size. Therefore, a parathyroid gland that is visible on ultrasound is usually abnormal. When occasionally visualized, normal parathyroid glands supposedly appear as small, smooth oval structures, isoechoic or hypoechoic to the thyroid gland measuring 5–6 mm in length and 3–4 mm in width. Superior parathyroid glands are posterior to middle or upper portion of the thyroid gland and inferior parathyroid are posterior and inferior to the lower pole of thyroid gland. Parathyroid adenomas, the most common parathyroid lesion, typically appear as well- defined discrete oval masses that are homogeneously hypoechoic relative to the thyroid gland with a thin echogenic rim (Fig. 3.36.1). An echogenic thyroid capsule may be visible separating the parathyroid lesion from the thyroid gland. Less commonly, parathyroid lesions may be bilobed or multilobulated especially in parathyroid hyperplasia. Inferior parathyroid glands located deep to the clavicles become visible by having the patient swallow during the real-time observation and by intentional inferior-oblique scan planes. Atypical ultrasound features include heterogeneous echotexture, isoechogenicity, cystic changes and calcification which occur due to fibrosis, haemorrhage, cystic degeneration and fat deposition. Larger lesions tend to have atypical ultrasound appearance when they outgrow the vascular supply. Parathyroid carcinoma is larger in size (>3 cm), have higher prevalence of atypical features and may show frank infiltration of adjacent structures. In the absence of frank invasion or abnormal outlines, these mimic parathyroid adenomas, and the diagnosis is usually made on histology. On colour Doppler, a characteristic extrathyroidal feeding vessel (typically a branch of the inferior thyroidal artery) is seen entering the parathyroid gland at one of the poles. The artery feeding the adenoma tends to branch around the periphery of the gland before penetrating deeper, resulting in a characteristic arc or rim of vascularity (Fig. 3.36.1). Parathyroid lesions need to be measured in three dimensions – length, width and the breadth to have a better correlation with the postoperative weight. The location with respect to the thyroid gland, major adjacent vessels (CCA and IJV) and also the distance between the skin surface and the lesion must be mentioned in the report to facilitate surgical planning. The diagnostic performance of ultrasound in the localization of abnormal parathyroid gland is operator dependent. In the hands of experienced radiologist, the sensitivity can reach as high as 92%. However, ultrasound as a modality has an overall pooled sensitivity of 76% (95 CI = 65%–81%). With increased use of high-resolution ultrasound, incidental detection of parathyroid lesion in asymptomatic patients is not uncommon and constitutes 1% of parathyroid adenomas, which are usually detected in a young patient. The need for treatment in this setting is determined after assessing the biochemical profile and the bone density of the patient. Pitfalls and limitations of parathyroid ultrasound: Ultrasound elastography objectively evaluates tissue hardness by measuring tissue elasticity. Parathyroid lesions being softer than thyroid nodules have supposedly characteristic speckled appearance on shear wave elastography using ARFI technology. The virtual touch quantification (VTQ) values are significantly different between the thyroid and the parathyroid lesions with parathyroid lesions having lower shear wave velocity values than thyroid lesions and the normal thyroid gland. These differences may help better differentiate parathyroid lesions from its mimics. However, its role in routine clinical practice is dubious, and the results are not consistently reproducible and hence its routine application is limited in daily sonographic practice. Ultrasound-guided FNAC is not performed routinely. But in select patients with clinically suspected primary hyperthyroidism with intrathyroidal parathyroid adenoma and those with atypical ultrasound appearance, ultrasound-guided FNAC is indicated. The parathormone levels in the aspirate are measured for confirmation of diagnosis. Ultrasound-guided FNAC is also performed for parathyroid lesions more than 3 cm in the largest dimension to exclude the possibility of parathyroid carcinoma. The purpose of the parathyroid scintigraphy is to preoperatively localize one or more hyperfunctioning parathyroid glands to achieve high cure rates for minimally invasive parathyroid surgery. Since the 1990s, Tc-99m MIBI (sestamibi) replaced thallium (Tl-201) and Tc-99m tetrofosmin as the radiopharmaceutical of choice for parathyroid imaging due to the superior imaging characteristics. MIBI (hexakis-2-methoxyisobutyl isonitrile) is a lipophilic cation member of the isonitrile family. The mitochondria-rich overactivated oxyphil cells in a pathologically enlarged parathyroid gland result in increased tracer uptake, longer retention and slower clearance of the tracer when compared with normal thyroid and parathyroid glands, and this phenomenon is used for identifying the abnormal parathyroid gland.
3.36: Imaging of parathyroid glands
Introduction
Embryology and anatomy of the parathyroid glands
Superior
Inferior
Undescended superior
Undescended in a submandibular location
Parapharyngeal
Thymus
Tracheooesophageal groove
Thyrothymic tract/ligament
Paraoesophageal
Carotid sheath
Posterosuperior mediastinum
Anterosuperior mediastinum
Carotid sheath
Intrathyroidal
Intrathyroidal
Pericardium
Pathology, biochemical findings and clinical presentation
Imaging modalities in primary hyperparathyroidism
Ultrasonography
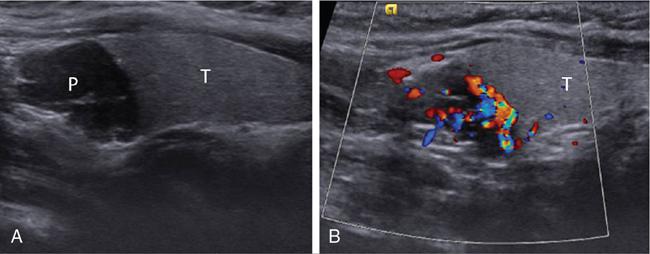
Ultrasound elastography in hyperparathyroidism
Ultrasound-guided fine needle aspiration cytology
Nuclear scintigraphy
Types of imaging
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree