The main neonatal stroke syndromes discussed in this article are: arterial ischemic stroke (AIS), including perinatal AIS, and “presumed” perinatal AIS; cerebral venous thrombosis, including cortical vein and venous sinus thrombosis and germinal matrix hemorrhage/periventricular hemorrhagic infarction; and intraparenchymal hemorrhage. This review discusses general pathophysiological mechanisms and the role of imaging in these conditions.
The main neonatal stroke syndromes discussed in this review are: arterial ischemic stroke (AIS), including perinatal arterial ischemic stroke (PAIS) and “presumed” perinatal AIS; cerebral venous thrombosis (CVT), including cortical vein and venous sinus thrombosis and germinal matrix hemorrhage/periventricular hemorrhagic infarction; and intraparenchymal hemorrhage. Cerebral infarction in neonates may also occur as a result of cerebral hypotension causing hypoxic-ischemic brain injury, multiple vascular occlusions in meningoencephalitis and cerebritis, and in some rare congenital disorders such as incontinentia pigmenti. Infarctions due to abnormal metabolism at a cellular level may occur in neurometabolic disorders such as molybdenum cofactor deficiency, organic acidemias, and hypoglycemia. These other causes of cerebral infarction are beyond the scope of this article.
The World Health Organization defines stroke as “a clinical syndrome of rapidly developing focal or global disturbance of brain function lasting more than 24 hours or leading to death with no obvious nonvascular cause.” This definition of stroke as a clinical syndrome with acute onset of a neurologic deficit is not readily applicable to strokes occurring in early life, either in children or in neonates. It is recognized that by this definition, children will have a nonvascular cause for their neurologic presentation in 1 out of 3 cases. Furthermore, neonates with an acute stroke may be asymptomatic, particularly preterm infants, or they may have a nonspecific clinical presentation such as lethargy, hypotonia, apnea, or feeding difficulties. The published series that have reported the highest rates of neonatal stroke are those in which the diagnosis was made by detecting an infarct on neuroimaging performed during routine screening rather than in symptomatic infants. For these reasons the diagnosis of stroke in neonates, as in children, relies on radiologic (or pathologic) confirmation.
It is also recognized that some strokes occur in utero before birth. In the published literature perinatal stroke has been inconsistently defined as a focal cerebrovascular insult sustained at any time between 20 and 28 gestational weeks and 7 to 28 days of neonatal life. In an attempt to reach some consensus of definition, at least for the purposes of research, the National Institute of Child Health-National Institute of Neurological Disorders and Stroke (NICH-NINDS) perinatal workshop defined perinatal stroke as a “a group of heterogeneous conditions in which there is focal disruption of cerebral blood flow secondary to arterial or cerebral venous thrombus or embolization” occurring “between 20 weeks of fetal life through the 28th postnatal day and confirmed by neuroimaging or neuropathological studies.” Their imaging criteria defined stroke as either a partial or complete occlusion of a vessel with a focal brain lesion in that territory or brain imaging corresponding to infarction in a recognized vascular territory. By this definition an acute neurologic presentation is no longer necessary, while neuroimaging has become essential in making the diagnosis of perinatal stroke.
Some children in whom stroke is not detected in the neonatal period may present later with signs such as asymmetry of reach and grasp, failure to reach normal milestones, postnatal seizures, and congenital hemiplegia. In these children, in whom the presentation of hemiplegia or seizures is not acute, the diagnosis of presumed perinatal AIS is made based on neuroimaging appearances of a chronic arterial territory infarct. Hence the timing of the AIS is remote from the clinical presentation. It is assumed to be perinatal on the basis that beyond the neonatal period, stroke is likely to have presented as an acute focal or global neurologic deficit, and also on the presence of characteristic imaging features that establish that the stroke most likely occurred in early life.
The NICH-NINDS classification separates perinatal stroke into 3 groups: (1) fetal ischemic stroke diagnosed before birth using imaging or in stillborns on the basis of postmortem pathologic examination, (2) neonatal ischemic stroke diagnosed after birth and before the 28th postnatal day (including preterm infants), and (3) presumed perinatal ischemic stroke, diagnosed in infants older than 28 days in whom it is presumed (but not certain) that the ischemic event occurred sometime between the 20th week of fetal life through the 28th postnatal day.
Cranial ultrasonography, computed tomography (CT), and magnetic resonance (MR) imaging are the 3 main imaging techniques available to image the neonatal brain. Ultrasonography is readily available, inexpensive, portable, and can be performed at the bedside. It is usually the first-line brain-imaging technique performed on the neonatal unit, and allows serial examinations to be performed without transfer of the sick neonate to the radiology department. However, ultrasonography lacks sensitivity for all types of neonatal stroke, particularly lesions at interfaces with bone such as posterior fossa lesions and peripherally based cortical lesions. It is dependent on the level of skill and experience of the operator. CT has some advantages over MR imaging in that it is a quicker examination with less need for sedation. CT depicts venous sinus thrombosis and hemorrhage well. However, potential concerns regarding radiation effects on the developing neonatal brain and increased lifelong risk of cancer limit its use to neonates who are acutely deteriorating clinically and in whom neurosurgical intervention is being considered, or when there is limited access or contraindication to MR imaging. Multislice brain CT should be performed according to low-dose pediatric protocols that vary dose according to weight and age. The authors do not recommend the use of currently commercially available portable CT scanners for imaging the neonatal or infant brain because diagnostic quality is less good and a greater radiation dose is required compared with departmental scanners. Overall, CT is less sensitive for the detection and characterization of brain lesions, and MR imaging is the modality of choice for evaluating the neonatal brain. However, it is important that MR imaging and sedation protocols are optimized to allow multiplanar imaging, multiple sequences (T1, T2, and diffusion-weighted imaging) providing differing tissue contrasts, and vascular imaging (MR angiography, MR venography) without artifacts from motion.
Perinatal arterial ischemic stroke
Incidence
Eighty percent of perinatal stroke is attributable to AIS; this differs from the relative incidence of AIS in children in whom ischemic stroke (AIS and cerebral sinovenous thrombosis [CSVT]) and hemorrhagic stroke occur with the same frequency. The incidence of perinatal AIS is estimated to occur in around 1 in 1600 to 5000 births in populations from the United Kingdom, Switzerland, the United States, and Estonia. The United Kingdom study found that acute PAIS was more common in boys than in girls whereas presumed PAIS was more common in girls.
These variations in incidence may be explained by differing diagnostic criteria and use of MR imaging, as well as different study populations. For example, the Estonian study included all neonatal stroke, including hemorrhagic stroke, in its cohort. Both this and the United States study included retrospectively diagnosed, presumed PAIS as well as neonatal AIS. These two studies had the highest incidences and showed that many perinatal strokes (42%, 68% of all PAIS) are not diagnosed until later in life. This figure may still be an underestimate of the true incidence of PAIS, as it is likely that some PAIS is asymptomatic or associated with very mild deficits and therefore may go undetected during life. A hospital-based study by Benders and colleagues found that PAIS is not uncommon in preterm infants under gestational age of approximately 34 weeks, with an even greater incidence of 7 in 1000. Two possible explanations for this relatively high incidence may be the use of routine cranial ultrasonography in this population and their exposure to more invasive procedures during their stay in the neonatal intensive care unit.
Perinatal stroke affects 20 to 62.5 per 100,000 live births. This high incidence compares with an annual incidence rate of stroke in children after the first month of life of 2.3 to 13 per 100,000 per year, similar to the incidence of pediatric brain tumor. The peak incidence of AIS is in the first year of life, and does not rise to these levels again until much later in life. The risk is highest in the perinatal period soon before birth and in the month after. This increased risk of neonatal AIS is mirrored by a parallel increased risk of ischemic stroke in the mother that is up to 5 times greater just before and for the day after delivery than earlier in pregnancy or when not pregnant. This increased maternal and neonatal risk of ischemic stroke is related to the activation of coagulation cascades during normal birth, likely to be an evolutionary adaptation to reduce the risk of blood loss during delivery.
Pathophysiology and Risk Factors
The majority of PAIS is most likely to be caused by thromboembolism passing from the placenta through the patent neonatal foramen ovale, although other potential sources include the fetal/neonatal heart and extracranial vessels ( Box 1 and Fig. 1 ). By the end of the embryonic period the developing cerebral arterial system is already similar anatomically to that of the adult, and therefore the arterial territories that are affected in PAIS are the same as those in adults. Most PAIS occurs in the middle cerebral artery (MCA) territory, typically either a complete MCA infarct or a posterior truncal infarct, and the left MCA is affected 3 to 4 times more commonly than the right. This bias has been explained as the result of hemodynamic arterial flow; placental emboli or systemic venous emboli in the fetus or neonate may pass through a patent foramen ovale or patent ductus arteriosus directly into the left common carotid artery and hence to the left MCA. The sick newborn is also at risk from right-to-left shunting associated with respiratory disease, pulmonary hypertension, or congenital heart disease.
Maternal factors
Autoimmune disorders
Coagulation disorders (Protein C deficiency, Protein S deficiency, Factor V Leiden)
Anticardiolipin antibodies
Twin-to-twin transfusion syndrome
In utero cocaine exposure
Infection
Placental factors
Placental thrombosis
Placental abruption
Placental infection
Fetomaternal hemorrhage
Cardiac disorders
Congenital heart disease
Patent ductus arteriosus
Pulmonary valve atresia
Cardiac surgery (associated with cardiac bypass, atrial balloon septostomy)
Fetal and neonatal blood and lipid disorders
Polycythemia
Disseminated intravascular coagulopathy
Factor V Leiden mutation
Protein S deficiency
Protein C deficiency
Prothrombin mutation
Homocysteine
Lipoprotein(a)
Factor VIII
Infection
Central nervous system infection
Systemic infection
Vasculopathy
Vascular malformation or defect
Trauma and catheterization
Birth asphyxia
Dehydration
Extracorporeal membrane oxygenation

Hence most of the associated risk factors for perinatal stroke appear to be related to an increased propensity for thromboembolism. The physiologic activation of the coagulation cascade in the fetus and mother around delivery is normally a transient phenomenon, which may explain why the recurrence risk of neonatal stroke is extremely low, and then mainly seems to recur in children in whom there is an underlying procoagulation defect. This situation differs from that in AIS in children, in whom the recurrence risk is 6% to 13% with an even higher recurrence risk of transient ischemic attack (TIA) or “silent infarct.”
While thromboembolism is also a risk factor in pediatric stroke, it appears there is a greater range of underlying etiological factors for stroke in children; arteriopathy is one of the commonest causes of childhood stroke and is associated with recurrence risk, a feature that has not been recognized (or systematically studied) in neonatal stroke. Beyond a few case reports in which the diagnosis of dissection was presumptive and made without pathognomonic radiological evidence of a dissection flap or intramural thrombus, there is little evidence that arterial dissection is a common cause of PAIS, unlike in older children.
Risk factors for thromboembolism for PAIS include fetal/neonatal, maternal, and placental factors, and are found in 42% to 78% of PAIS. In one study thromboembolic risk factors were found in 68% of PAIS compared with 24% of normal controls. Multiple risk factors for thromboembolism may coexist. Normal neonates already have several risk factors for thrombus formation in the perinatal period, including a raised hematocrit, presence of fetal hemoglobin, high procoagulant proteins, and increased blood viscosity. Additional blood and lipid disorders are also associated with PAIS. Twin pregnancies have a greater risk of PAIS, which appears to be independent of twin-twin transfusion syndrome or co-twin demise.
Congenital heart disease is an independent risk factor for perinatal stroke. White matter lesions are more common than arterial territory cortical infarcts. In studies investigating brain MR imaging abnormalities in children with congenital heart disease, periventricular white matter injury was seen on MR imaging in 16% to 43% of patients with congenital heart disease prior to any operative procedure. The changes were focal and asymptomatic, and were seen in children without any acute postnatal hypoxic-ischemic event. Although established lesions do not appear to progress postoperatively, additional ischemic lesions are found after cardiac surgery. Impairment of brain development during the fetal period in children with congenital heart disease (measured by quantitative advanced MR imaging techniques) is recognized. It is suggested that the brains of children with congenital heart disease are immature and behave in a similar way to the brains of preterm babies affected by hypoxic-ischemic injury, and that the white matter is particularly susceptible. In these studies risk factors associated with acquired postoperative brain injury included risk factors for cerebral hypotension, such as cardiopulmonary bypass (CPB) with regional cerebral hypoperfusion, lower intraoperative cerebral hemoglobin oxygen saturation during the myocardial ischemic period of CPB, and low mean blood pressure during the first postoperative day. However, neonates with congenital heart disease are also at risk of AIS. Preoperative AIS is typically arterial territory branch cortical infarction and is associated with balloon atrial septostomy, whereas white matter injury is not. Children are also at risk of thromboembolic AIS from cardiac catheters and other vascular catheters. Extracorporeal membranous oxygenation (ECMO) is a specific thromboembolic risk. Specific artificial devices such as the Berlin Heart, a pediatric mechanical ventricular assist device, are also associated with neonatal AIS ( Fig. 2 ).

Although an association with hypoxic-ischemic encephalopathy (HIE) in term babies has been described, there is actually little evidence for HIE as a cause when strict diagnostic definitions are used. The more usual scenario is a term baby born in good condition after an uncomplicated pregnancy and labor or elective cesarean section. Hypoglycemia is recognized as an independent risk factor for PAIS in preterm babies but not in term babies, in whom hypoglycemic brain injury usually manifests as bilateral parieto-occipital lobe infarction.
The placenta itself has an important role in the etiology of thromboembolism in perinatal stroke; placental disorders include thrombosis, abruption and fetomaternal hemorrhage, placental infection, and chorioamnionitis. Placental thrombotic vasculopathy is a commonly recognized finding, and may be linked to maternal prothrombotic conditions. Emboli may also arise from umbilical vascular catheters.
Maternal factors associated with PAIS include maternal procoagulation tendencies and autoimmune disorders. Factor V Leiden deficiency, increased lipoprotein(a) and antiphospholipid antibodies, and heterozygosity or homozygosity of methyltetrahydrofolate reductase mutations are seen with greater frequency in neonates with PAIS and their mothers. Other maternal factors include a history of infertility, infection, preeclampsia, maternal trauma, diabetes, in utero cocaine use, prolonged labor, and instrumented delivery. Primiparity has been identified as a risk factor in 30% to 75% of cases of PAIS in term infants but not in preterms. This finding may be more closely related to a prolonged second stage of labor, as primiparity did not remain a statistically significant independent risk factor on multivariate analysis.
Clinical Presentation
Acute presentation in the neonatal period
Although epidemiologic data may be less accurate given the limitations of missed or later diagnoses, approximately 60% of cases of PAIS do present acutely in the neonatal period, mostly with recurrent focal seizures in the first 3 days of life. Therefore about 40% of the children do not have specific symptoms in the neonatal period, and are only recognized later with the emergence of motor impairment, developmental delay, specific cognitive deficiency, or seizures. Twenty-five% to 40% of term infants with PAIS present with seizures. After hypoxic ischemia, perinatal arterial ischemic stroke is the second most common cause of neonatal seizures in term newborns ; this differs from AIS in childhood whereby the most common presentations are an acute focal deficit (91%), seizures (23%), or headache (44%).
Typically PAIS occurs in a term baby with a normal antenatal course who appears generally well. The baby usually has a normal neurologic examination with preserved primitive reflexes, and no or minimal signs of encephalopathy. Usually the seizures in PAIS are focal with clinical recovery in between, matched by electroencephalography (EEG) changes of focal spikes and/or sharp waves during seizures, but usually a normal background EEG. In other neonates there may be subtle, nonspecific neurologic signs of hypotonia, lethargy, poor feeding, duskiness, or apnea. Hemiparesis is rarely found on neurologic examination at this age. Hence the diagnosis of PAIS is made only when neuroimaging confirms a lesion consistent with focal infarction in an arterial vascular territory.
Presumed diagnosis of PAIS made retrospectively
Infants and children with presumed PAIS are diagnosed after the neonatal period; they do not have clinical signs during the neonatal period or may have signs that are so subtle that they escape detection. Such patients may present later with hemiplegia, focal hand weakness, or pathologic early hand preference occurring when younger than 1 year of age. These motor deficits are usually only detected from the middle of the first year of life onward, when coordinated voluntary motor activity is developing. Children may present with other long-term neurologic problems such as cognitive impairment and seizures. In these children the diagnosis is presumptive, and relies on confirmation of neuroimaging findings of a chronic arterial territory infarct.
Fetal or preterm ischemic stroke
Fetuses may be diagnosed with AIS based on a routine early second-trimester anomaly screening ultrasound scan (ideally confirmed by MR imaging), on ultrasound scans done later in pregnancy for other reasons, or on postmortem examination. PAIS in preterm infants is more likely to be asymptomatic than in term babies, and is often detected as the result of routine cranial ultrasonography or brain MR imaging in the (sick) preterm infant. In one study PAIS was detected in 10% of all preterms who had routine cranial ultrasonography as part of their assessment. Equally PAIS was found in 10% of preterms who were symptomatic with apneas that were subsequently confirmed as seizures using amplitude-integrated EEG.
Imaging
The role of imaging is to make or confirm the diagnosis of PAIS and to exclude stroke mimics such as encephalitis, hypoglycemia, and hypoxic ischemia. Neuroimaging can be used to help time the onset of the infarct and to confer information regarding prognosis. Neuroimaging definition criteria for PAIS are: (1) imaging evidence of a partial or complete occlusion of an artery in relation to a focal brain lesion, or (2) distribution of parenchymal lesion(s) that can only be explained by occlusion of a specific brain artery.
Site
The developing arterial system is already similar to the mature arterial system by the end of the embryonic period, the major differences being in the watershed sites between arterial territories, which have shifted from the germinal matrix and periventricular regions to more lateral parasagittal cortex by term. Hence the lesion pattern caused by occlusion of a particular artery is already established by 20 gestational weeks and is consistent throughout life.
Any arterial territory may be involved; however, most infants with PAIS show involvement of the MCA territory (75%) and usually of the left hemisphere (55%). Posterior circulation infarction is relatively unusual, and this is comparable with the distribution in children in whom isolated anterior circulation infarcts (73%) are much more common than posterior circulation infarcts (21%). Multiple arterial territories may be involved in thromboembolic disease, and 6% to 25% of perinatal arterial ischemic strokes are bilateral. The most common MCA branches affected in term infants are the main branch of the MCA, resulting in complete MCA territory infarction, and the posterior MCA trunk, causing posterior temporal and parietal lobe infarction. After this, in decreasing order of frequency, are infarcts in the territories of the internal carotid, anterior cerebral, posterior cerebral, posterior communicating, and anterior choroidal arteries. As in the term population, the majority of strokes in preterm infants involve the MCA (81%); however, the involvement of different branches of the MCA appears to change with gestational age. Involvement of one or more lenticulostriate branches was most common among infants with a gestational age of 28 to 32 weeks.
Evolution of infarction on neuroimaging
Magnetic resonance imaging
Swelling of astrocytes is seen on pathologic studies within 30 minutes of onset of ischemia, and by 4 to 6 hours swelling of oligodendrocyte nuclei and cytoplasm is seen microscopically. MR imaging, particularly diffusion-weighted imaging, is more sensitive than both cranial ultrasonography and CT for the detection of acute AIS, and MR imaging is the modality of choice in the neuroimaging evaluation of suspected neonatal stroke.
Animal experiments show restricted diffusion on brain MR imaging scans within the infarcted parenchyma minutes after ischemia. The exact mechanism accounting for the appearances of restricted diffusion in acute ischemia is not completely understood. In tissue ischemia energy depletion leads to disruption of the energy-dependent ion pump, which causes increased intracellular sodium and water and results in cytotoxic edema. There is a net shift of water from the extracellular space to the intracellular space, with reduced free water movement within the interstitial fluid. This shift is not the only contribution to the signal hyperintensity on diffusion-weighted images, as increased signal due to T2 effects becomes more important later when cytotoxic edema has already occurred.
On diffusion-weighted imaging the earliest changes of infarction are seen as signal hyperintensity in the affected arterial territory, and can be seen before changes on T2-weighted sequences are appreciated. The signal hyperintensity is maximal until 4 days after birth and slowly declines, but remains higher than in normal brain for up to 1 to 2 weeks. These earliest diffusion-weighted signal hyperintensity changes are matched by low signal on calculated apparent diffusion coefficient (ADC) maps. The ADC values in infarction progressively decrease over the first 3 days before beginning to increase again, reaching normal values, or pseudonormalization, by 4 to 10 days. It is suggested that this ADC change broadly correlates with the presence of cytotoxic edema. Thereafter, ADC values progressively increase as vasogenic edema develops. The evolution of these changes with time is probably similar to those changes described in adults though has not been systematically studied. However, the signal hyperintensity seen on diffusion-weighted images in adults often persists for longer, often appearing hyperintense weeks after the initial event. In adults the cortical gray matter shows a smaller drop in measured ADC values and a slightly faster rate of increase in ADC than white matter, hence pseudonormalization occurs slightly earlier in the cortical gray matter. This appearance is also probably similar in neonates.
The cortex in the region of infarction is initially hyperintense on T2-weighted imaging for the first 6 days. It is seen as loss of the normal, relatively darker signal of the cortical ribbon in comparison with the unmyelinated white matter ( Fig. 3 ). On T1 weighted imaging the cortex may appear relatively dark with respect to normal cortex. During this time the white matter also appears hyperintense on T2-weighted imaging, and this persists for 2 to 3 weeks or so before declining to eventually appear similar to unaffected brain. The white matter appears mildly hyperintense on T1-weighted imaging during the first 8 to 9 days compared with the normal cerebral white matter (though not as bright as the T1 shortening seen with subsequent haemorrhagic transformation or cortical highlighting). This feature is particularly apparent in acute neonatal AIS, possibly because of the relatively greater contrast between the normal low signal of the neonatal unmyelinated cerebral white matter and adjacent cortex.

Neonates have smaller vessels with lower blood flow velocities than children or adults, making MR angiography and MR venography of the neonatal brain more technically challenging for assessment of the vascular anatomy, vessel diameter, and flow. Three techniques are available: time-of-flight, phase-contrast, and contrast-enhanced MR angiography. Each has its advantages and disadvantages, but the first 2 techniques may be successfully used in the neonate without the need for contrast. In PAIS, MR angiography may show medium-vessel or large-vessel occlusion, supporting the pathogenesis of thromboembolism ( Fig. 4 ), but may also be normal.

Ultrasonography
Although relatively insensitive for the diagnosis of AIS, cranial ultrasonography is usually the first neuroimaging test that will be performed, either in symptomatic term infants or on routine screening of sick preterm infants. The typical appearances are a well-defined wedge-shaped region of increased echogenicity affecting cortex and white matter in a recognized arterial territory. Doppler studies in MCA infarcts may, or may not, show transient reduced flow and pulsatility on the affected side that becomes similar to the contralateral unaffected side by 24 hours. The exact reason for this is not known; possible explanations for the subsequent improved flow include migration of arterial embolus and compensatory cross-flow. Most neonatal strokes have a patent MCA by the time of presentation.
The sensitivity of ultrasonography for the detection of AIS increases with the age of the infarct (68% sensitive in the first 3 days compared with 87% between days 4 and 10 ) but it is well recognized to miss small, peripherally located cortical infarcts, and posterior circulation infarcts, even when these are retrospectively diagnosed following a positive MR imaging study. Repeated imaging may be required to detect a lesion. Typically an MCA infarct is easier to diagnose when mass effect is at its greatest, around the third day, where ultrasonography may have some advantage over MR imaging is in the detection of small perforator territory AIS such as those seen in preterm infants.
Necrosis and organization
From 6 hours to 6 days coagulation necrosis is associated with damage to the endothelium and breakdown of the blood-brain barrier, leading to vasogenic edema and brain swelling. Swelling of the affected brain is usually maximal at 3 days and is evidenced by sulcal effacement, and in large anterior circulation infarcts by subfalcine and uncal herniation with shift of midline structures. If the cerebral swelling progresses, contralateral hydrocephalus may develop. Although not routinely given, contrast enhancement may be seen as a consequence of disruption of the blood-brain barrier. Hemorrhage may be seen as the result of reperfusion of the infarct. Increased flow velocities such as diastolic arterial flow with a lowered resistance index and increased venous flow may be seen on ultrasonography as a result of regional luxury perfusion.
The pathologic description of laminar necrosis is of necrosis in the cortex; this may affect all layers or just the middle to deeper cortical layers (pseudolaminar necrosis). The imaging correlate of this is believed to be cortical highlighting, or T1 shortening localized to the cortex. This is seen from days 5 to 6 after onset; from this time the cortex also becomes dark on T2-weighted imaging These cortical changes are generally thought to be related to petechial hemorrhage or increased paramagnetic substances, release of myelin lipids, or calcification. Susceptibility-weighted imaging has been used in attempts to differentiate the changes seen on T1-weighted imaging from hemorrhage; in one study the cortical highlighting did not correspond with dark signal on susceptibility imaging, and the assumption was made that the changes are not due to hemorrhage. However, this study did not differentiate extracellular met as in methemoglobin hemoglobin, which is bright on both T1-weighted and T2-weighted sequences, from the other molecular forms of hemorrhage that do demonstrate susceptibility effects.
From 3 days to 6 weeks the infarct organizes, a process in which central liquefaction develops in necrotic areas with gliosis, breakdown of myelin, microcyst formation, calcification, and peripheral neovascularization. During this time the infarct shows signal changes in keeping with increased water content compared with unaffected brain, but has not yet cavitated. Strands of tissue may be seen crossing the infarct.
Tissue loss
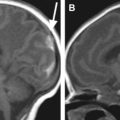
Cystic cavitation and atrophy in the affected arterial territory are seen from approximately 4 weeks after onset. Typically the cyst is lined by a gliotic scar, and there may be hemosiderin. On MR imaging this is seen as regions of cystic cavitation or encephalomalacia with surrounding signal change in keeping with gliosis. When imaged later in life, as in a presumed large perinatal MCA infarct the whole of the cerebral hemisphere appears smaller, and there may be compensatory expansion of the calvarial diploic space and paranasal and mastoid sinuses ( Fig. 5 ).

Acute secondary neuronal pre-Wallerian and Wallerian degeneration
Secondary signal intensity changes remote from the infarction are seen in the brainstem and thalamus in the first week. In some MCA infarcts, restricted diffusion occurs outside the MCA territory within the more caudal ipsilateral corticospinal tract within the brainstem ( Fig. 6 ). These diffusion changes occur earlier than the signal hyperintensity on T2-weighted images and focal swelling seen subsequently. Within a few weeks these changes progress to mature injury with evidence of atrophy and gliosis, sometimes with T1 shortening, as a secondary neuronal degenerative or Wallerian-type phenomenon. Similarly, acute changes of restricted diffusion with subsequent T2 signal changes and swelling are also seen within the medial thalamus, which are likely attributable to secondary degeneration of corticothalamic projections. Contralateral cerebellar hemisphere atrophy may be seen as a result of crossed cerebellar diaschisis following MCA infarction.

Treatment
Although there are currently 3 international evidence-based stroke guidelines for the investigation and management of stroke in children, only the American Heart Association (AHA) Stroke Council guideline (2008) specifically addresses neonatal stroke. There is no specific treatment for stroke, and management is supportive and directed at modification of etiological factors as well as treatment of the complications of stroke such as epilepsy. Recommendations for surgery are ventricular drainage of hydrocephalus complicating hematoma evacuation or shunting if hydrocephalus persists (Class I recommendation), and hematoma evacuation in the presence of raised intracranial pressure, though this may not necessarily improve outcome (Class II recommendation). Anticoagulation may be considered for recurrent thromboembolic stroke, but specific recommendations are not made. Thrombolytic agents are not recommended in neonates while more information about the safety and effectiveness of these agents is awaited.
Predicting Outcome
The main adverse outcomes following PAIS are hemiplegia, epilepsy, visual impairment, cognitive impairment, and behavioral difficulties. However, some children (33%–40%) will have a normal outcome, presumably due to plasticity of the neonatal brain. There is no evidence of an increased risk of death in neonates with AIS unless there are additional complicating factors such as congenital heart disease or HIE. All of the outcome studies to date show that the outcome for babies with stroke is better than that for older children and adults.
Motor Impairment
Up to 50% of neonates with PAIS will develop subsequent hemiplegia. Hemiplegia is usually not evident in the neonate or infant, but may be detected in later life. Children with PAIS may also develop more subtle motor impairments, which may present even later. Nevertheless, most children will still be able to achieve independent walking. Early MR imaging performed around the time of the acute infarct can be used to predict the subsequent development of hemiplegia. In general, small infarcts of the posterior cortical branches of the MCA or posterior cerebral artery are associated with a better prognosis than large main-branch MCA infarcts. Involvement of 3 sites of cerebral infarction (basal ganglia, posterior limb of the internal capsule, and temporoparietal cortical infarction) as seen in main-branch MCA infarction predicts future hemiplegia, whether occurring at term or preterm. Acute pre-Wallerian secondary neuronal degeneration with restricted diffusion in the corticospinal tract caudal to the infarct also predicts future hemiplegia. Combining these findings further refines motor outcome prediction. In their study of 73 infants with PAIS affecting the MCA territory, Husson and colleagues found that 72% of babies with superficial cortex, basal ganglia involvement, and restricted diffusion of the corticospinal tract (CST) developed later motor impairment by age 2 years. Mixed infarctions involving superficial cortex and basal ganglia ( P <.001) and CST involvement ( P <.001) were highly predictive of hemiplegia, whereas most babies (88%) with isolated superficial cortical infarcts did not develop future motor impairment. Absence of CST involvement predicted normal motor outcome in 94%.
The risk of epilepsy for a child with PAIS increases with time, and PAIS is a common cause of epilepsy in children referred for consideration of epilepsy surgery. Epilepsy may be seen in up to 50% of children, whereas cognitive outcome is more frequently impaired in PAIS than in childhood stroke, and ranges from 25% to 40%. However, the success of early brain MR imaging in predicting subsequent epilepsy, cognitive outcome, and behavioral difficulties is more limited. Some studies suggest that cognitive impairment is worse when there are more extensive main-branch infarcts involving superficial cortex and deep gray matter. Other studies suggest that cognitive outcome is more closely associated with hemiplegia and epilepsy than extent of MCA lesion, and may occur with smaller infarcts when there are additional neonatal factors. Preterm infants with PAIS had more associated problems, such as language delay, than term infants.
Recurrence Risk
The recurrence risk for stroke appears to be very low. The Childhood Stroke Study Group followed up 215 neonates with arterial ischemic stroke for a median time of 3.5 years, during which time 7 children (3.3%) developed symptomatic thromboembolism (AIS, CSVT, or deep vein thrombosis of the leg). Four children developed a second AIS (∼2%). Five children had a prothrombotic risk factor, and the second event was often triggered by a specific underlying problem such as infection, cardiac abnormality, or moyamoya disease.
Cerebral sinovenous thrombosis
Incidence
The Canadian registry, one of the largest cohorts of pediatric stroke, estimated the incidence of cerebral venous thrombosis as at least 0.67 per 100,000 children per year, whereas a German study estimated this at a higher rate of 2.6 per 100,000. Neonates were the most frequently affected age group and accounted for 43% of cases in the Canadian registry. The estimated incidence of neonatal CSVT was 40.7 per 100,000 live births per year. This figure is likely to be an underestimate of the true incidence because the clinical presentation is nonspecific, the diagnosis may be missed clinically in the presence of coexistent illnesses occurring in the sick neonate, and the radiological diagnosis itself is not always straightforward, though it is easier to make in the acute phase. Not all CSVT is recognized to be symptomatic, and some CSVT clearly remains undiagnosed in neonatal or later life. In some cases the diagnosis is made on the basis of imaging performed for other reasons. A high index of suspicion is required to make the clinical and radiological diagnosis.
Pathophysiology and Risk Factors
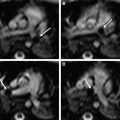
CSVT has been associated with all the thrombotic risk factors that have been implicated in PAIS, such as Protein C or Protein S deficiency, G20210A prothrombin mutation, factor V Leiden mutation, antiphospholipid antibodies, infection, and polycythemia. Prothrombotic abnormalities have been reported in 15% to 20% of neonates with CSVT. A recent study comparing presumed PAIS and venous infarction in neonates found that there were no differences in prothrombotic conditions between the two groups. However, children with presumed perinatal AIS were more likely to have acute perinatal risk factors (66% vs 17%, P = .002) including fetal distress, emergency cesarean section, or neonatal resuscitation. This finding is supported by a meta-analysis of 1764 patients in published observational studies, which reviewed the impact of thrombophilia on risk of first childhood stroke. A statistically significant association with first stroke was demonstrated for each thrombophilia trait evaluated, with no difference found between AIS and CSVT. Venous sinus thrombosis and venous infarction may coexist with AIS in the same patient ( Fig. 7 ).
In CVST there is often a history of an acute neonatal illness, for example an infection, meningitis, or dehydration, and other comorbidities such as neonatal congenital heart disease are common. Associated acute systemic illnesses at the time of diagnosis were present in the majority (61%–84%) of cases. Five percent of all infants with ECMO have evidence of CSVT. Complicated delivery is a common finding in CSVT, and traumatic delivery may disrupt the superior sagittal sinus or cortical veins. Maternal factors such as maternal diabetes and preeclampsia are also recognized.
The lesions detected in cerebral venous thrombosis are thrombus within an occluded or partially occluded vein or venous sinus, venous ischemia, venous infarction, and hemorrhage. Following venous sinus thrombosis there is retrograde transmission of raised venous pressure proximal to the level of venous obstruction. This process increases both the venular and capillary hydrostatic pressure, which results in leakage of the capillary fluid into the interstitial space, causing vasogenic edema. The fluid leakage is often accompanied by red blood cells and is the usual cause of hemorrhagic venous infarcts in CSVT. Most of the edema is vasogenic and hence reversible but, if the process progresses, the capillary hydrostatic pressure and interstitial pressure can exceed the arteriolar pressure. This process can sometimes cause true venous infarction in which impairment of both arterial inflow and venous outflow occur.
Clinical Presentation
The diagnosis of CSVT should be considered in neonates presenting with seizures and encephalopathy, but the presentation may be more subtle and nonspecific and may include lethargy, apnea, and poor feeding, much like PAIS in this period. Around half of neonates present within the first 2 days of life and another 25% in the first week of life. Seizures may be subtle and focal or generalized. Some infants have relatively mild symptoms of encephalopathy despite extensive thrombosis within the venous sinuses. It is also recognized that venous sinus thrombosis may be detected as an incidental finding on brain MR imaging performed for other reasons.
Imaging
CSVT may be detected and missed on all imaging modalities, and in general isolated cortical vein thrombosis is not reliably and consistently diagnosed on any modality. However, because of the greater coverage achievable of the deep and superficial venous structures and superior evaluation of the brain parenchyma, CT and MR imaging are preferable to ultrasonography. MR imaging remains the modality of choice, as it does not involve ionizing radiation and is the most sensitive technique for detecting parenchymal lesions.
On unenhanced CT in the acute stage the involved venous sinuses appear hyperdense and expanded, the “dense triangle” or “cord sign” ( Fig. 8 ). It can sometimes be difficult to discriminate between normal appearances of the neonatal venous sinuses, in which blood appears relatively hyperdense compared with the adjacent brain ( Fig. 9 ). This normal appearance in the neonate is the result of a combination of persistent fetal hemoglobin and raised hematocrit; together they increase electron density in blood within the venous sinuses and hence increase the attenuation of x-rays. In addition, there is greater contrast between the relatively hyperdense normal neonatal venous sinus and the relatively low-density unmyelinated neonatal brain. The use of contrast, either a delayed postcontrast CT of the brain or a CT venogram, may facilitate the detection of nonenhancing thrombus, the “empty delta” or “empty triangle” sign.
On MR imaging a combination of T1-/T2-weighted sequences in multiple planes, as well as MR venography, are often necessary to make the diagnosis and avoid artifacts. Reliance on a single sequence, including MR venography, may lead to underdiagnosis or overdiagnosis ( Fig. 10 ). One observation and potential pitfall of 2-dimensional time-of-flight MR venography in neonates is that there seem to be more gaps in flow in the venous sinuses, particularly the posterior aspect of the superior sagittal sinus, which has been attributed to the age-related smaller size of the sinus, reduced venous flow, and skull molding.