Fig. 13.1
Grip techniques used in rock climbing. Closed cling grip (a), open cling grip (b), crack climbing (c), open grip (d), pinch grip (e), and pocket grip with one finger (f)
13.2.4 Open Grip
13.2.5 Cling (or Crimp) Grip
Cling (or crimp) grip is obtained with the fingers flexed 90° or more at the PIP joint and slightly extended at the DIP joint (Jebson and Steyers 1997). This grip can be open or closed, depending on the position of the thumb (Kubiak et al. 2006). In a closed grip, the thumb pushes over the index finger, allowing the thumb to be used as an extra holding force. This method of recruiting the thumb makes it a favorite grip for climbers faced with small holds, but it is regarded as the most painful grip (Logan et al. 2004). Indeed, it is associated with very high tensile force in the flexor tendons and places tremendous demands on the annular pulley system (Marco et al. 1998; Moor et al. 2009; Moutet 2003; Schoffl et al. 2003, 2004a, b, 2007a; Vigouroux et al. 2008). The A2 pulley must sustain 40 % more tension than the underlying tendon. This is the grip commonly associated with pulley ruptures and chronic thickening (Bollen 1988).
13.2.6 Pocket Grip
Pocket grip involves placing one or two fingers (the middle finger usually included, as it is the strongest) in small holes (Jebson and Steyers 1997). It is another particularly demanding grip because, during ascension, the flexor tendons support most, if not all, of the climber’s body weight. This grip is commonly associated with flexor digitorum profundus avulsions (Kubiak et al. 2006).
13.2.7 Pinch Grip
Pinch grip is used for small bone prominences to obtain a pinching action with the thumb and index finger (Martinoli et al. 2005). In the vertical (or slope) grip, both DIP and PIP joints are bent and pull down on the hold. This grip is also useful when holding one’s hand in rock fissures.
13.2.8 Crack Climbing
Finally, crack climbing involves wedging the fingers or the entire hand in cracks of varying sizes and depths (Jebson and Steyers 1997). If climbers slip off the rock with their feet, sudden high torsional forces are applied to the fingers, which may lead to fractures, ligament tears, or even dislocation of the finger joints (Schweizer 2012). Additionally, it has been suggested that crack climbing may be associated with a high incidence of carpal tunnel syndrome, due to the sustained position of wrist flexion.
13.3 Skin Injuries
Skin lesions are relatively frequent in rock climbers. Fingertip injuries include maceration and splitting of the skin on the finger pads due to prolonged pressure and abrasion (Cole 1990; Lapègue et al. 2010). Both mechanical factors and ischemic mechanisms can cause epidermal breakdown (Jebson and Steyers 1997). Hypertrophic scar tissue forms on the palmar surface of the hand in response to the repetitive abrasion and wear that usually occurs with crack climbing (Cole 1990; Jebson and Steyers 1997).
13.4 Flexor Tendon Injuries
13.4.1 Anatomy
The digital flexor tendons pass through the carpal tunnel before spreading out in the palm toward their respective fingers. Each finger has two flexor tendons: the flexor digitorum superficialis (FDS), which inserts on the mid-portion of the middle phalanx, and the flexor digitorum profundus (FDP), which inserts on the volar base of the distal phalanx. The FDS tendon splits at the distal metacarpal, passes around the FDP tendon, and reunites deep to the FDP tendon at the level of the PIP joint. Thus, the FDS tendon forms a ring aperture through which the FDP tendon passes to become the superficial tendon at the level of the shaft of the proximal phalanx (Moutet 2003). From the neck of the metacarpal to the DIP joint, the flexor tendons are lined by a synovial sheath that provides nutrition and lubrication to the tendons. Nutrition of the dorsal face of the FDP is also provided by the vinculas, slender, tendinous bands whose integrity may also prevent or decrease the FDP displacement when it is avulsed.
13.4.2 Injuries: Generalities
The flexor tendons are the most frequently affected tendons. They are particularly susceptible to injury when performing the cling and pocket grips (Kubiak et al. 2006). These maneuvers place excessive stress on the tendons and surrounding structures. With the cling grip, the majority of the stress of weight bearing is transferred from the hyperextended DIP joint to the flexed PIP joint along the FDS tendon. Because the cling grip is used most frequently, the FDS tendon is the one most likely to be injured (Jebson and Steyers 1997).
13.4.3 Tendinopathy and Tenosynovitis
In flexor tendinopathy and tenosynovitis, the patient experiences pain and swelling along the palmar surface of the digit, possible extending into the palm or forearm. While the patient’s passive flexion is normal, active flexion is usually limited (Jebson and Steyers 1997). Ultrasound findings include effusion, tendon and tendon sheath thickening, and hyperemia (Fig. 13.2). If MRI is performed, the liquid is depicted within the tendon sheath with high signal intensity on fluid-sensitive sequences. In some cases, synovial thickening and debris can be encountered in the tendon sheath as a result of synovial proliferation. The distinction between tenosynovial fluid and synovial proliferation can be facilitated by the administration of intravenous contrast material in conjunction with MRI. A patient with flexor tendinopathy or tenosynovitis should rest, take anti-inflammatory medication, and do range-of-motion exercises. Corticosteroid injection is rarely used but may be indicated in patients with chronic tendinitis or tenosynovitis in whom all other treatment modalities have failed. The injection should be performed carefully, as intratendinous injections may result in tendon rupture (Brinks et al. 2010; Jebson and Steyers 1997).
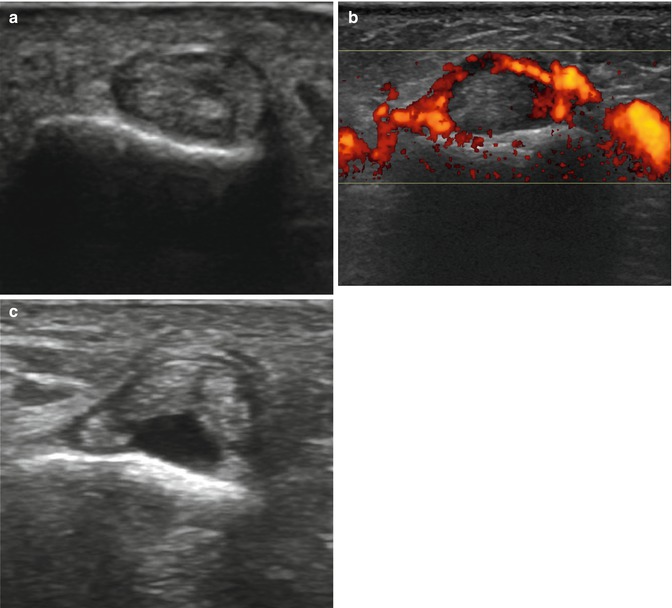

Fig. 13.2
Flexor tendons assessed with ultrasound. Transverse ultrasound images show normal flexor tendons (a), tenosynovitis of the flexor tendons with hyperemia of the tendon sheath on Doppler energy (b), and rupture of the flexor digitorum profundus tendon (c)
13.4.4 Nodules
Patients with a history of repetitive flexor tendon strains may present a palpable nodule in the digit or distal palm. The nodule is located within the tendon itself and may cause locking or triggering of the digit. During digital extension, the nodule catches on the first annular (A1) pulley, resulting in a triggering sensation (Jebson and Steyers 1997). If the nodule increases in size, it may eventually become too large to slide beneath the pulley, resulting in a “locked” finger that cannot be extended either actively or passively. If a nodule is present, it may be palpated within the flexor tendon upon physical examination. Triggering may be reproduced by applying pressure over the A1 pulley during flexion and extension of the involved digit (Jebson and Steyers 1997). Ultrasound shows segmental swelling of the flexor tendon (generally FDS), which may have a focal hypoechogenic nodule with loss of the fibrillar pattern and a peritendinous hypoechoic rim. Dynamic ultrasound depicts the conflict between the nodule and the pulley.
Treatment includes injection of a corticosteroid and lidocaine hydrochloride preparation into the flexor tendon sheath. If triggering continues even after two injections administered at an interval of at least 6 weeks, or if a patient’s digit is locked, surgical release of the A1 pulley is indicated (Jebson and Steyers 1997). Ultrasound-guided steroid injections for trigger finger disease seem to be a more efficient alternative (Hansen and Lange 2013).
13.4.5 Avulsion and Rupture
FDS tendon rupture typically occurs with the cling grip and FDP tendon rupture with the pocket grip (Bannister and Foster 1986). Patients complain of acute onset of pain when performing a grip. Clinical findings include tenderness at the FDS or FDP tendon insertion, digital swelling, and an absence of active flexion of the PIP joint (with an FDS tendon rupture) or DIP joint (with an FDP tendon rupture, which is a Jersey finger lesion). The end of the tendon frequently retracts, and tenderness and swelling may consequently also be noted more proximally in the digit or even in the palm. The degree of tendinous retraction is influenced by an associated lesion of the vincula (for the FDP) and by the size of a potential bone fragment. In the case of the Jersey finger, there is a five-step classification depending on the vincula lesion, level of retraction, presence of a bone fragment, and other associated fracture(s) (Lapègue et al. 2010).
Plain films may be helpful in the rare cases where bone avulsions occur at the attachment of the flexor tendons or in the case of associated fractures (Lapègue et al. 2010). Ultrasound can easily demonstrate a complete rupture, characterized by focal loss of the fibrillar appearance of the tendon, replaced by a hypo- or anechoic aspect or by non-visualization of the tendon, blunt torn ends, refractive shadowing, and adjacent fluid (Lapègue et al. 2010) (Fig. 13.2). The description of the location of the retracted proximal tendon facilitates appropriate surgical access (Lapègue et al. 2010). Moreover, real-time scanning can evaluate tendon gliding. Passive dynamic maneuvers allow better visualization of the tear and demonstrate the lack of mobilization of the proximal tendon (Lapègue et al. 2010). MRI also demonstrates the location of the distal end of the retracted tendon, especially in the sagittal plane (Bencardino 2004; Lapègue et al. 2010; Ragheb et al. 2005; Rubin et al. 1996). Coronal fluid sequences with or without fat suppression can show a distended “serpiginous” tendon retracted at the palm and surrounded by edema. FDS tear, which is quite a rare lesion, can be associated with a tear of the volar plate of the PIP and a concomitant rupture of the FDP (Lapègue et al. 2010).
13.5 Pulley Injuries
13.5.1 Anatomy of the Pulley System
In each finger, the flexor tendons are maintained against the phalanges by a fibrous digital sheath, whose thickness varies along the length of the finger in order to allow flexion movements at the interphalangeal joints. These thickenings are the annular pulleys and the cruciform bands (Martinoli et al. 2005) (Fig. 13.3). The annular pulleys are transverse, well-defined structures located at five specific points and numbered from proximal to distal (A1–A5) (Figs. 13.3 and 13.4). The A1 pulley extends from the area of the palmar plate of the MCP joint to the base of the proximal phalanx; the A2 pulley extends from the cranial part of the proximal phalanx to the junction of the proximal two-thirds and the distal third of the proximal phalanx; the A3 pulley is small and located over the PIP joint; the A4 pulley lies in the middle third of the middle phalanx; and the A5 pulley is located over the DIP joint (Martinoli et al. 2005; Moutet 2003). Three cruciform pulleys (C1–C3) form an additional system of criss-crossing fibers between the A2 and A3 pulleys (C1), the A3 and A4 pulleys (C2), and the A4 and A5 pulleys (C3). They are thinner and more flexible than the pulleys and have a less important effect on digital performance (Martinoli et al. 2005).
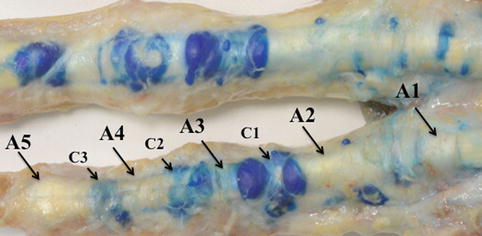
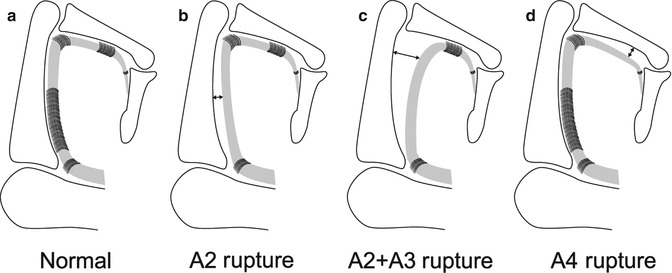

Fig. 13.3
Annular (A1–A5) and cruciform (C1–C3) pulleys. Picture shows cadaver dissection of the digit pulleys after injection of a blue-tinted gel into the flexor tendons sheet

Fig. 13.4
Schematic representation of pulley’s injuries. Drawing shows (a) normal pulleys, (b) complete A2 pulley rupture, (c) complete rupture of A2 and A3 pulleys, (d) complete A4 pulley rupture
13.5.2 Physiology
The main function of the annular pulleys is to attach the tendon sheaths to the bony skeleton, thus stabilizing the tendon during finger flexion and avoiding palmar “bowstringing.” The cruciform pulleys are designed to permit deformation of the tendon sheath during flexion without impingement on the tendon itself (Moutet 2003).
Physiological studies (Bollen 1990; Lin et al. 1989; Marco et al. 1998; Rohrbough et al. 2000) have demonstrated that, of the five annular pulleys, the A2 pulley (mean length 16.3 mm) and the A4 pulley (mean length 5.8 mm) are the broadest and most important in resisting the palmar displacement of the flexor tendons. A2 is the strongest and A4 the stiffest.
13.5.3 Injuries
In rock climbers, A2 pulley rupture is a relatively common injury (up to 40 % of professional climbers (Bollen and Gunson 1990; Jebson and Steyers 1997)) (Fig. 13.4). The A2 tears more commonly than the A4 pulley, and its rupture may occur alone or in association with the A3 pulley, whereas the A1 pulley always remains intact. From a biomechanical point of view, the cling grip has often been associated with A2 pulley injury because of the sharp angle position of the tendons and the amount of hyperextension that this position requires (Gabl et al. 1998). The middle and ring fingers are most commonly involved (Vigouroux et al. 2008).
Pulley rupture can occur acutely or develop insidiously. In acute rupture, the patient reports a sudden sharp pain over the volar aspect of the affected finger, often associated with an audible “pop” at the proximal phalanx, followed by soft tissue swelling and local ecchymosis (Marco et al. 1998). The area is tender to palpation, and visible and palpable bowstringing of the flexor tendons is usually noted during active resisted finger flexion. If the trauma is more significant, simultaneous rupture of more than one pulley may occur with various combinations of A2-A3 and A2-A3-A4 pulley lesions and more evident bowstringing of the flexor tendons (Marco et al. 1998) (Fig. 13.4). Expansion of pulley damage may also occur in climbers who continue to climb when already injured. Selective A4 pulley tears can be observed either in the crimp position or with a significant overload on a bent DIP joint while the PIP joint is extended (Lin et al. 1989; Marco et al. 1998; Martinoli et al. 2005). Bowstringing of the FDP across the PIP joint with resisted flexion of the fingertips is considered a diagnostic sign for a tear of the A2 pulley (Gabl et al. 1998), but this feature may be concealed by soft tissue swelling and pain in the acute phase.
Overall, ultrasound has proved to be 98 % sensitive, 100 % specific, and 99 % accurate in diagnosing (Martinoli et al. 2005) pulley ruptures and can be considered the gold standard for such an assessment. Only in case of doubt should an additional MRI be performed. With high-resolution probes, ultrasound is able to identify the normal annular pulleys as very thin (thickness 0.3–0.5 mm (Klauser et al. 1999)) anisotropic bands covering the flexor tendons. On transverse planes, the superficial portion of the pulleys has a hyperechoic, fibrillar appearance related to the perpendicular incidence of the ultrasound beam, whereas its lateral portions are hypoechoic as a result of anisotropy (Klauser et al. 2005) (Fig. 13.5). Dynamic scanning during flexion and extension movements of the fingers can help distinguish the fixed pulleys from the underlying gliding tendons.
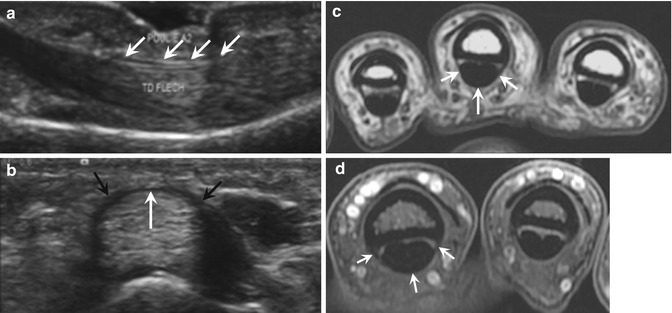

Fig. 13.5
Normal A2 pulley. (a) Sagittal ultrasound image opposite the first phalanx showing a thin hypoechoic line above the flexor tendons corresponding to the A2 pulley (arrows). (b) On the transverse ultrasound image, the A2 pulley appears as a thin band covering the flexor tendons, with a typical fibrillar appearance on its volar portion (white arrow), and extending down to the bone on both sides (black arrows). Axial T1- (c) and T2-weighted fat-suppressed (d) MRI show the A2 pulley as a thin hypointense band (arrows) which is better visualized at its sites of insertion into the proximal phalanx than in its volar portion
In annular pulley tears, ultrasound diagnosis is essentially based on the demonstration of subluxation of the flexor tendons that, instead of coursing along the concavity of the phalanges, lie at a variable distance from the bone. A value of 1.0 mm should be considered as the upper limit for the distance between the flexor tendon and phalanx with forced flexion for a normal pulley (Lapègue et al. 2010; Klauser et al. 2002a). Establishing which pulley is ruptured can be achieved by referring to the site of maximal volar bowstringing along the long axis of the finger. In A2 pulley rupture, the maximal volar displacement occurs over the proximal phalanx during forced flexion (<3 mm and ≥3 mm in incomplete and complete rupture, respectively) (Fig. 13.6). Complete combined rupture of the A2 and A3 pulley is associated with a tendon displacement ≥5 mm opposite those pulleys. In A4 pulley tears, bowstringing is observed over the middle phalanx (≥2.5 mm in complete rupture) (Lapègue et al. 2010; Martinoli et al. 2005).
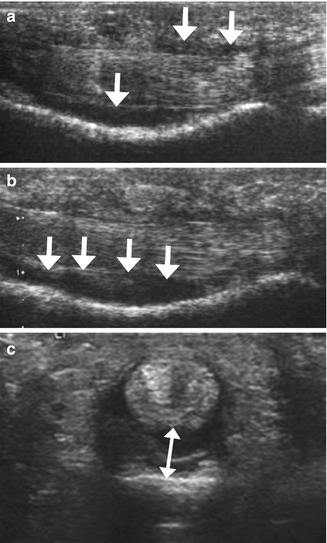

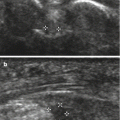
Fig. 13.6
Complete A2 pulley rupture. Longitudinal ultrasound images demonstrate a flexor tendons/proximal phalanx distance of A2 (a) increasingwith forced flexion against resistance (b) with more fluid collecting deep to the flexor tendons (arrows). Transverse ultrasound image (c) shows associated tenosynovial effusion posterior to the flexor tendons (double arrow)
In pulley ruptures, sheath fluid can be observed in the acute phase as a result of traumatic tenosynovitis. The distribution of sheath fluid differs in acute tenosynovitis and in pulley rupture: in acute tenosynovitis, the fluid tends to expand superficial to the flexor tendons because these tendons are compressed against the phalanges by intact pulleys; in pulley tears, most of the fluid collects deep to the flexor tendons in the area of the affected pulley because of the free space left over the bone by their displacement (Fig. 13.6).
Incomplete pulley ruptures are more difficult to diagnose because they cause only mild tendon displacement or none at all. However, the affected pulley appears swollen and hypoechoic (Martinoli et al. 2005). Fibrous tissue underneath the tendon has also been reported in asymptomatic climbers and may represent posttraumatic reparative change (Klauser et al. 1999).
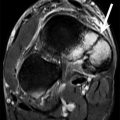
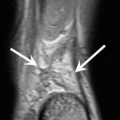
MRI is able to image the pulleys directly as focal thickenings of low signal intensity located superficial to the flexor tendons (Ragheb et al. 2005) (Fig. 13.5). This imaging modality provides better delineation of the pulley attachments into the cortex of the phalanges than ultrasound. In suspected annular pulley tears, an appropriate MRI technique includes positioning the finger in extension and forced flexion (Parellada et al. 1996) while asking the patient to press his fingertip against the pulp of his thumb. The protocol usually includes thin sagittal T1- and T2-weighted sequences. Some authors add transverse gadolinium fat-saturated T1-weighted sequences because this best assesses the abnormal A2 pulley (Goncalves-Matoso et al. 2008). Correlative imaging of the adjacent normal fingers enhances diagnostic confidence. Established MRI criteria for complete and incomplete pulley tears are mainly related to the grade and location of tendon dislocation and to the visualization of the disrupted or absent pulley (Bencardino 2004). On sagittal images, bowstringing extending from the PIP joint to the base of the proximal phalanx indicates complete rupture of the A2 pulley (Fig. 13.7). Bowstringing extending from the PIP joint but not as far as the base of the proximal phalanx indicates incomplete rupture of the A2 pulley. Bowstringing extending from the base of the proximal phalanx into the area distal to the PIP joint indicates complete combined A2 and A3 pulley rupture. Bowstringing extending from the base to the middle part of the intermediate phalanx indicates complete A4 pulley rupture (Klauser et al. 1999, 2000, 2002a, b, Klauser et al. 2005). Additional signs of pulley rupture include the visualization of underlying fluid in the area of the affected pulley and local soft tissue edema or hematoma (Fig. 13.7).
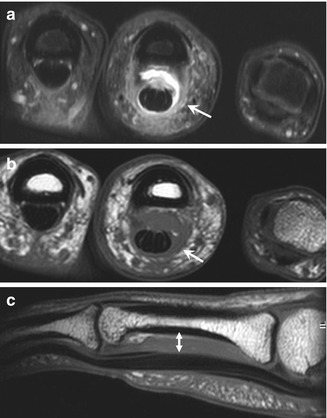

Fig. 13.7
Complete A2 pulley rupture. Axial fat-suppressed T2-weighted MRI (a) of the ring finger shows hyperintense edema and hemorrhage between the flexor tendons and the proximal phalanx as a result of a complete A2 pulley tear. On the axial fat-suppressed T2- (a) and T1-weighted (b) MRI, the thickening and blurred aspect of both lateral components of A2 (arrow) with increasing signal confirm the rupture. Sagittal T1-weighted MRI (c) demonstrates an anterior tendon displacement in the area of the A2 pulley (double arrowhead)
Other features related to overuse may be associated: thickening of the pulleys (Fig. 13.8) and joint capsule, small cysts in close proximity to the pulleys, thickened flexor tendons (Klauser et al. 1999), cortical hypertrophy and finally degenerative joint disease, including subchondral cysts and osteophytes (Martinoli et al. 2005).
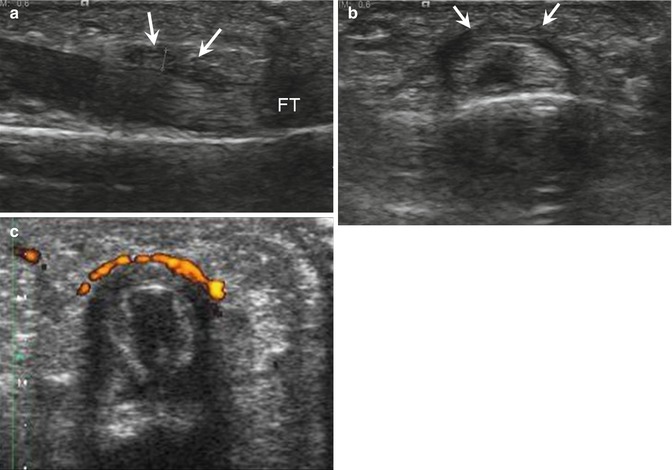

Fig. 13.8
A 27-year-old climber with A2 pulley thickening, presenting with pain of the right index finger for 2 months. Longitudinal (a) and transverse (b) ultrasound images show a thickened hypoechoic A2 pulley (arrows) above the flexor tendons (FT) with strong hyperemia on power Doppler (c)
Some authors have proposed a diagnostic and therapeutic algorithm in suspected pulley injury (Schoffl et al. 2003; Schoffl and Schoffl 2006). In high-level professional climbers, the ruptured pulleys can be reconstructed to restore efficient biomechanical characteristics of the flexor apparatus. Patients who are treated conservatively or who refuse surgery usually tape their fingers between the joints to reinforce the flexor tendon pulley system so as to avoid worsening of the existing lesions (Martinoli et al. 2005). If there is any uncertainty regarding the diagnosis of A2 pulley rupture or the management of this type of injury, referral is recommended.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree