Recent advances in imaging have allowed a better understanding of imaging features and classification of vascular anomalies. This article focuses on imaging of vascular malformations; describes the updated classification system and clinical and imaging characteristics of the different subtypes; and discusses the associated syndromes, differential diagnosis, and available treatment options, including the role of imaging in management.
Key points
- •
The latest classification from International Society for the Study of Vascular Anomalies (ISSVA) divides vascular malformations into simple, combined, vascular malformations of named major vessels and syndromic vascular malformations.
- •
Simple vascular malformations include high-flow and low-flow vascular malformations.
- •
Low-flow vascular malformations include venous malformations, capillary malformations, and lymphatic malformations.
- •
High-flow vascular malformations include arteriovenous malformations and arteriovenous fistulae.
Introduction
Vascular anomalies (VAs) are a group of lesions that result from errors of vascular embryogenesis. VAs include vascular tumors and vascular malformations, typically present during childhood, and may involve any part of the body. They often pose a diagnostic dilemma, because of the multiplicity of appearances, wide differential diagnosis, and the various classification systems that have been proposed over the years.
The International Society for the Study of Vascular Anomalies (ISSVA) provides the most standardized and accepted classification. The ISSVA classification was first introduced in 1997 and most recently updated in 2018. This classification divides VAs into 2 major categories: (1) vascular tumors, and (2) vascular malformations. , Vascular tumors are true neoplasms that show rapid postnatal growth and slow regression into late childhood; these include hemangiomata and kaposiform hemangioendothelioma. In contrast, congenital vascular malformations (CVMs) do not regress or enlarge; they grow at the same rate as the child and include arteriovenous malformations (AVMs), arteriovenous fistulae (AVFs), capillary malformations (CMs), venous malformations (VMs), and lymphatic malformations (LMs). In the current version of the ISSVA classification, CVMs are divided into 4 groups: simple malformations, combined malformations, malformations of major named vessels, and malformations associated with other anomalies (syndromic vascular malformation). Based on their flow characteristics, simple vascular malformations are further differentiated into high-flow lesions (AVM and AVF) and low-flow lesions (CM, VM, LM). The 2018 version of the ISSVA classification emphasizes the known genetic mutations associated with CVM, reflecting a growing interest in the genetic basis of vascular malformations ( Tables 1 and 2 ).
| VAs | ||||
|---|---|---|---|---|
| Vascular Tumors | Vascular Malformations | |||
| Simple | Combined | CVMs of major named vessels | Syndromic CVMs |
|
|
| See Table 2 | |
|
| |||
| ||||
| Clinical Syndrome | Vascular Malformation | Other Manifestations | Mutation |
|---|---|---|---|
| Sturge-Weber | CM; face, trigeminal nerve distribution | Gyral atrophy, glaucoma | GNAQ |
| Klippel-Trénaunay | CM, LM, VM; cutaneous and soft tissue | Overgrowth of soft tissue and bone, usually limited to 1 limb | PIK3CA |
| Parkes Weber | CM, VM, AVM; cutaneous and soft tissue | Overgrowth of soft tissue and bone, usually limited to 1 limb | RASA1 |
| Proteus syndrome | CM, VM, LM; soft tissue ± visceral | Disproportionate progressive overgrowth of hands or feet | AKT1 |
| Maffucci syndrome | VM; soft tissue | Multiple enchondromas in the hands and feet, ±chondrosarcoma | IDHH1/IDH2 |
| CLOVES syndrome | CM, VM, LM, AVM | Lipomatosis, nevi, scoliosis, skeletal anomalies hands and feet | PICK3CA |
Imaging
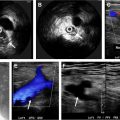
Gray-scale ultrasonography coupled with color Doppler imaging and spectral analysis is the initial screening imaging modality at some centers for vascular malformation given the low cost, fast and real-time information, and lack of ionizing radiation. However, ultrasonography is limited in its ability to accurately assess large and deep lesions and to detect bone involvement. Contrast-enhanced ultrasonography has been suggested for evaluation of CVMs and has been used for monitoring response to therapy, including microcirculatory changes.
Magnetic resonance (MR) imaging is the most valuable modality for imaging vascular malformation because of its high temporal and contrast resolution, which allows accurate assessment of CVMs and their relation to the surrounding structures. The ability to characterize flow dynamics by MR imaging permits differentiation of high-flow and low-flow CVMs and enables discrimination between CVMs and other vascular lesions. MR imaging has also been used for treatment planning and assessment of treatment success. A comprehensive MR imaging examination should include contrast-enhanced conventional MR imaging and dynamic contrast-enhanced MR angiography (MRA), such as time-resolved imaging of contrast kinetics (TRICKS) or time-resolved angiography with interleaved stochastic trajectories (TWIST). The protocol for vascular malformations at our institution includes triplanar T2-weighted images with fat saturation, precontrast axial T1-weighted images, and postcontrast triplanar T1-weighted images with fat saturation. Because flow characteristics (arterial, venous, both arterial and venous, none) are essential to the classification of vascular malformations, we obtain our dynamic contrast-enhanced images with a temporal resolution of 1 to 1.5 seconds to allow separation of arterial and venous phases. Other investigators have recommended optional three-dimensional arterial spin labeling sequences for hemangiomas and arteriovenous shunting as well, and diffusion-weighted imaging to rule out other lesions and tumors when the diagnosis is uncertain ( Table 3 ).
| Venous | Lymphatic | Capillary | AVM | AVF | |
|---|---|---|---|---|---|
| Appearance | Serpentine tubular structures or dilated channels | Septated lobulated mass, microcytic or microcytic | Skin-thickness lesion | No well-defined mass Enlarged feeding arteries and draining vein + nidus | No well-defined mass Enlarged draining vein, no nidus |
| T1WI | Intermediate | Low | Low | Intermediate | Intermediate |
| T2WI | High | High | Slight high | High | High |
| GRE | Intermediate | Low, intermediate, high | — | High | High |
| Flow voids on SE | No | No | No | Yes | Yes |
| Fluid-fluid levels | Uncommon | Common | No | No | No |
| Enhancement | No arterial or early venous enhancement, slow gradual diffuse enhancement on delayed images | Rim and septal enhancement if macrocystic; no significant or slight diffuse enhancement if microcystic | Faint subcutaneous enhancement | Early enhancement of enlarged feeding arteries and nidus with shunting to draining veins | Early enhancement of enlarged feeding arteries with shunting to draining veins, no nidus |
| Ultrasonography | Heterogeneous, compressible | Variable echogenicity, noncompressible | Subtle low echogenicity | Low echogenicity | Low echogenicity |
| Low Venous flow, or sometimes no flow | No or low flow in the septa Both arterial and venous flow in the septa | Low to moderate | High Arteries: high velocity, low resistance, spectral broadening Veins: pulsatile venous flow Nidus: turbulent flow | High Arteries: high velocity, low resistance, spectral broadening Veins: pulsatile venous flow |
| Others | Phleboliths | — | — | Calcifications | — |
Radiography and contrast-enhanced computed tomography (CT) are not routinely used for the diagnosis of a vascular malformation owing to the use of ionizing radiation and the lower temporal resolution compared with MR imaging; however, radiographs and CT may detect phleboliths, calcification, and bone involvement. CT may be used as an alternative imaging for patients who cannot undergo MR imaging.
Simple vascular malformations
Low-Flow Malformations
Capillary malformation
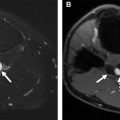
CMs, usually referred to as port-wine stain, are found in 0.5% of the population and affect the skin capillaries. They commonly present at birth as pink or red macules that do not involute. Most of these lesions involve the face and tend to be in the trigeminal nerve distribution. The lesion enlarges and darkens as a child grows older, resulting in a cobblestone appearance. Central nervous system (CNS) involvement can occur, particularly in association with lesions in the V1 or midline distribution. The risk of having Sturge-Weber syndrome increases to 25% when half of the face, including the ophthalmic division of the trigeminal nerve, is involved, and increases to 33% when both sides of the face are involved.
Most CMs can be diagnosed by a physical examination. With high-frequency ultrasonography transducers, CMs can appear as superficial hypoechoic areas that vary in depth from 0.2 mm to 3.7 mm, with increased vascularity on color Doppler imaging. , If MR imaging is performed, the lesion may appear as subtle signal abnormality within the subcutaneous fat with skin thickening. MR imaging and CT are helpful in evaluation for CNS involvement and may detect progressive gyral atrophy, gyral enhancement, enhancement of ipsilateral choroid plexus, and cortical calcification ( Fig. 1 ). Abnormal cortical veins and contrast stasis can be seen on cerebral angiography.