Fig. 10.1
Illustration of various types of tissue flaps. Fasciocutaneous (A). Musculomucosal (B). Myocutaneous (C). Bowel (D). Bone/osteomyocutaneous (E)
Fig. 10.2
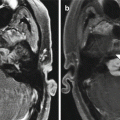
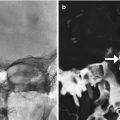
Temporalis flap. Axial (a) and coronal (b) T1-weighted MR images show the characteristic fan-shaped appearance of the flap that fills the right nasal cavity, maxillectomy cavity, and masticator space
Fig. 10.3
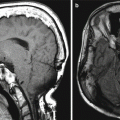
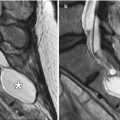
Fasciocutaneous rotation advancement flap. The patient has a large defect following Mohs surgery for a cutaneous malignancy of the left cheek. Axial T2-weighted MR images (a, b) demonstrate the Scarpa’s fascia component of the graft (arrows) as a low-signal-intensity band. The rest of the graft demonstrates normal fat signal intensity without evidence of recurrent disease. Atrophy of the left masticator muscles is noted
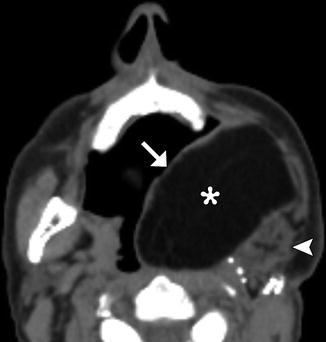
Fig. 10.4
Myocutaneous free flap. Axial CT image shows the skin (arrow), fat (*), and muscle (arrowhead) components of the thigh flap used to reconstruct the left oropharynx and oral cavity
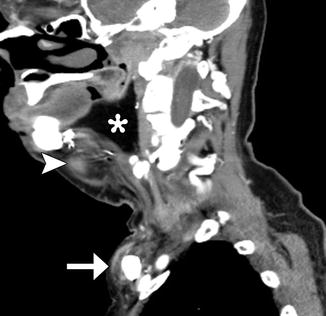
Fig. 10.5
Myocutaneous rotational flap. Axial CT image shows a pectoralis flap swung over the clavicle to fill a large surgical defect in the neck. The vascular pedicle is visible (arrow), as are the muscle (arrowhead) and adipose tissue (*) components of the flap. The muscle has undergone fatty degeneration

Fig. 10.6
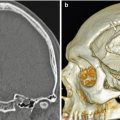
Osteomyocutaneous flap. Axial (a) and 3D (b) CT images show left maxillofacial reconstruction using a fibular graft (arrowheads) with surrounding soft tissues (arrows)
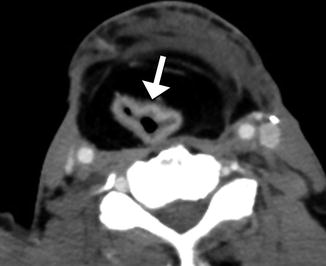
Fig. 10.7
Myocutaneous flap neopharynx. Axial CT image shows skin lining the neopharynx (arrow), which is surrounded by subcutaneous fat and muscle
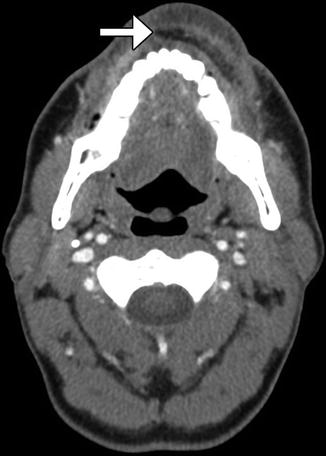
Fig. 10.8
Radial forearm free flap lip reconstruction. Axial CT image shows lower lip reconstruction utilizing radial forearm free flap and palmaris longus tendon (arrow), which provides near-anatomic contours

Fig. 10.9
Colonic interposition. Axial CT image shows a loop of large bowel (arrow) adjacent to the trachea
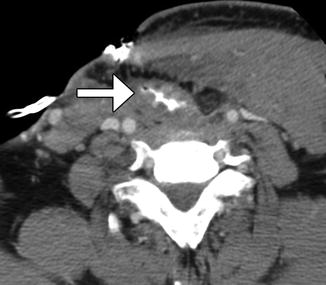
Fig. 10.10
Gastric transposition. Axial CT image shows the transposed stomach filled with barium, which outlines the rugal folds (arrow). Total pharyngolaryngoesophagectomy was also performed
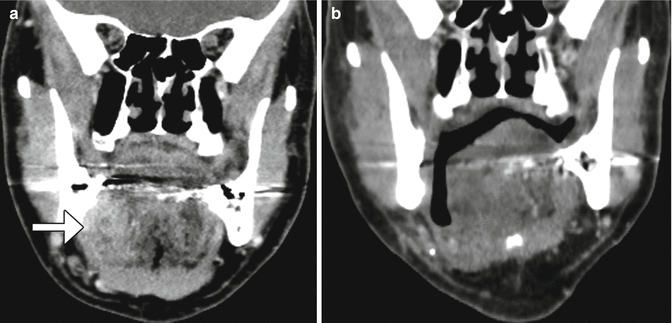
Fig. 10.11
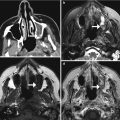
Facial artery musculomucosal (FAMM) flap. Preoperative coronal CT image (a) shows an infiltrative mass in the right floor of the mouth (arrow). Postoperative coronal CT image (b) shows interval resection of the mass and reconstruction with a flap that closely approximately approximates the floor of the mouth
Table 10.1
Types of flap reconstruction
Components | Type | Description |
|---|---|---|
Vascular supply | Random | Transected small vessels |
Free | Transected and reanastomosed large vessels | |
Pedicle | Intact vascular supply with tissue rotated into position | |
Tissue | Fasciocutaneous | Composed of subcutaneous fat and fascia as well as dermal/epidermal tissue |
Myocutaneous | Composed of muscle, subcutaneous, and dermal/epidermal tissue. They are used to close large soft tissue defects and may sometimes also be used to reconstruct the aerodigestive tract. Common donor sites include pectoralis major and the anterolateral thigh | |
Bone | Used to reconstruct mandible defects. Common donor sites include fibula, iliac crest, and scapula. May be used as part of an osteomyocutaneous flap | |
Bowel | Used for upper aerodigestive tract reconstruction and includes jejunal and ilial grafts and gastric and colonic interpositions | |
Musculomucosal | Used to close small to moderate upper aerodigestive tract mucosal defects, often using tissue supplied by the facial artery (FAMM flap) |
While serosanguinous fluid collections or seromas in the surgical bed after flap reconstruction are commonly encountered on early postoperative imaging, large perioperative hematomas are rare. However, these can lead to flap ischemia; therefore, prompt recognition and re-exploration can help salvage the flap. On CT, the hematomas can appear as mass-like heterogeneous lesions (Fig. 10.13).
Infection of the flap is a serious complication, which may require urgent debridement or revision. Diagnostic imaging is useful for delineating the extent of the infected fluid collections, which can contain gas, have peripheral enhancement, and surrounding fat stranding (Fig. 10.14). Infections in the surgical bed can be predisposed by the presence of fistulas with the skin and/or aerodigestive tract.
Anastomotic leaks are potential sources for infection. CT or fluoroscopic examinations with oral contrast administration can be useful for assessing the presence of leaks. The presence of hyperattenuation from the oral contrast material in extraluminal fluid collections is indicative of a leak (Fig. 10.15). A baseline CT without oral contrast can be useful for comparison, since surgically implanted hyperattenuating material can potentially be misinterpreted as contrast.
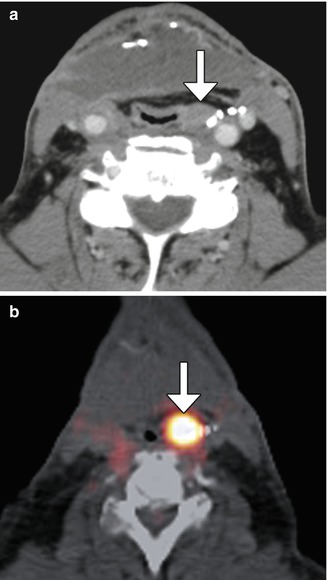
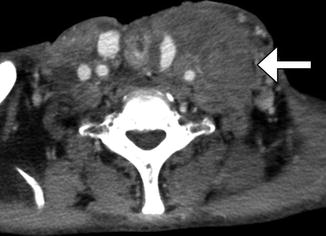
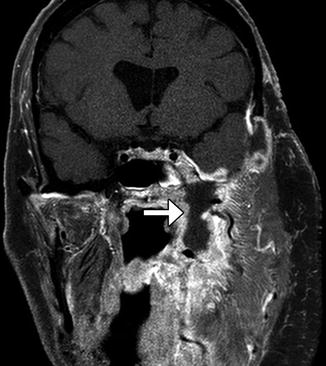
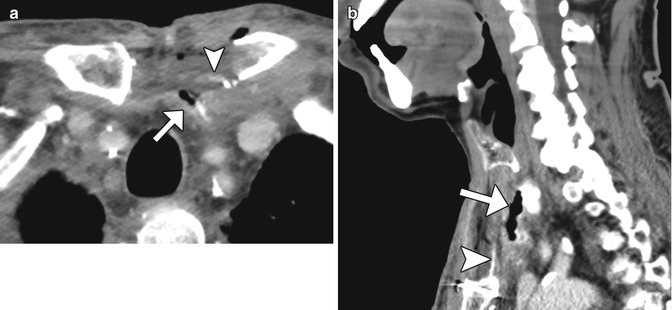

Fig. 10.12
Tumor recurrence. Axial CT image (a) shows a myocutaneous flap with subtle nodularity along the left aspect of the neopharynx (arrow) in a patient with history of head and neck squamous cell carcinoma, but no prior baseline exams available. 18FDG-PET/CT (b) was recommended and obtained 2 weeks later, which shows corresponding marked hypermetabolism (arrow)

Fig. 10.13
Perioperative hematoma. Axial CT image obtained shortly after laryngectomy shows a heterogeneous mass-like hematoma (arrow) underlying the edematous left pectoralis flap

Fig. 10.14
Infected flap. Coronal fat-suppressed post-contrast T1-weighted MRI shows a fluid collection with surrounding enhancement (arrow) deep to the left neck myocutaneous flap, which represents an abscess

Fig. 10.15
Anastomotic leak. Axial (a) and sagittal (b) CT images obtained with intravenous and oral contrast show the presence of extraluminal oral contrast (arrowheads) in a collection adjacent to the jejunal graft (arrows)
10.2 Neck Dissection
10.2.1 Discussion
Neck dissection is performed to diagnose and/or remove at risk and/or diseased lymph nodes and, in some cases, surrounding structures. The main types of neck dissection include selective, modified, radical, and extended neck dissection (Table 10.2). Features of each of these can be appreciated on CT (Figs. 10.16, 10.17, 10.18, 10.19, and 10.20).
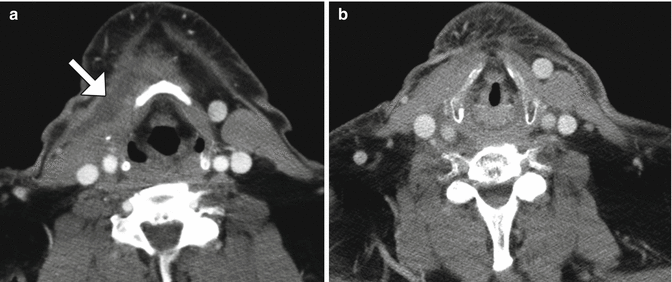

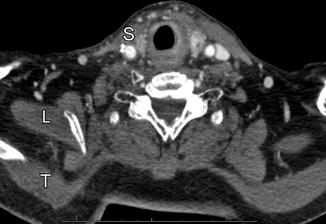
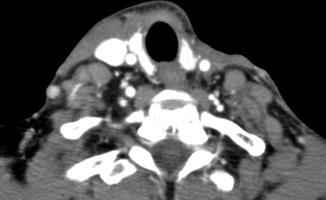
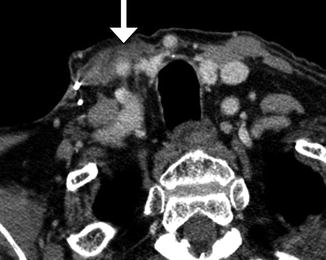
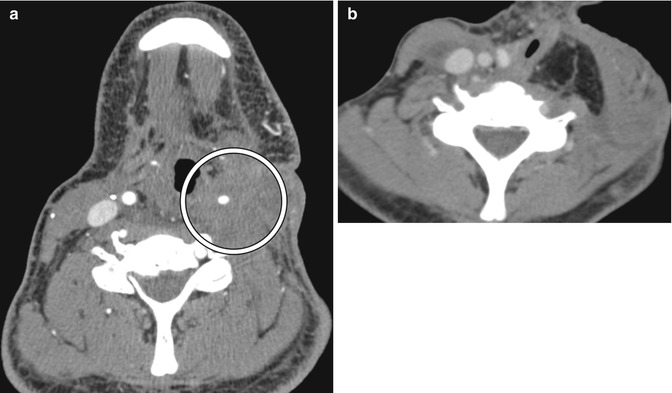
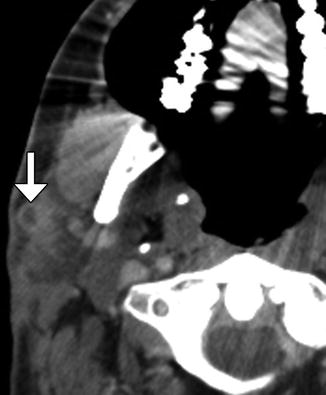
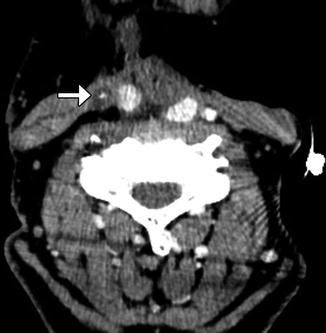
Table 10.2
Types of neck dissection
Type of neck dissection | Description |
|---|---|
Selective (Fig. A) 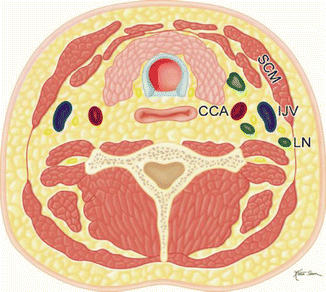 | Removal of selected lymph nodes between levels I and V with preservation of the sternocleidomastoid, internal jugular vein, and spinal accessory nerve intact. There are four main types of SND: supraomohyoid, anterior, posterolateral, and lateral |
Modified (Fig. B) 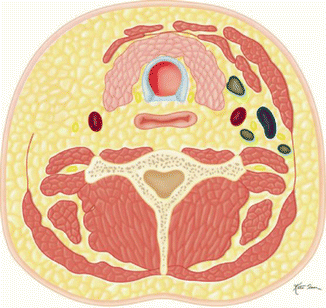 | Removal of levels I and V lymph nodes with preservation of the sternocleidomastoid, internal jugular vein, or spinal accessory nerve intact |
Radical (Fig. C) 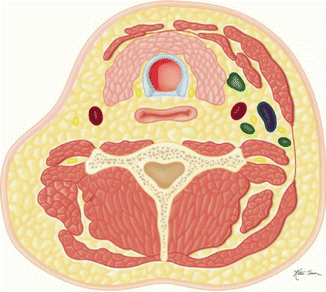 | Removal of selected lymph nodes from levels I and V, sternocleidomastoid, internal jugular vein, and spinal accessory nerve |
Extended (Fig. D) 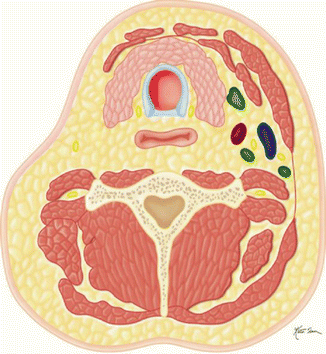 | Same as radical neck dissection along with removal of another lymph node group (i.e., superior mediastinal) or nonlymphatic structure (i.e., carotid artery) or structure not normally included in neck dissection (i.e., salivary gland, thyroid) |


Fig. 10.16
Selective neck dissection. Axial CT image 4 weeks after lateral neck dissection (a) shows a seroma (arrow) overlying the right sternocleidomastoid muscle. There is loss of fat surrounding the carotid artery and subcutaneous tissues. Axial CT image obtained 2 years after right lateral neck dissection and radiotherapy (b) shows subcutaneous stranding. The sternocleidomastoid muscle and internal jugular vein are intact. Axial CT image (c) shows resection of the right submandibular gland and remaining left submandibular gland (arrow), producing an asymmetric appearance that should not be confused with a mass lesion

Fig. 10.17
Modified radical neck dissection. Axial CT image shows the absence of the right internal jugular vein and atrophy of the sternocleidomastoid (S) and trapezius (T) muscles but compensatory hypertrophy of the right levator scapulae (L). There is also mild edema in the right neck soft tissues

Fig. 10.18
Radical neck dissection. Axial CT image shows the absence of the left sternocleidomastoid and internal jugular vein as well as concavity of the left neck contour

Fig. 10.19
Radical neck dissection with pectoralis rotational flap. Axial CT image shows right radical neck dissection, including resection of the right sternocleidomastoid. Instead, there is a pectoralis rotational flap (arrow) that covers the carotid artery

Fig. 10.20
Extended neck dissection. Preoperative axial CT image (a) shows an infiltrative tumor (arrow) that encases the left carotid artery. The patient had undergone prior radical neck dissection and radiation therapy. Postoperative axial CT image (b) shows interval sacrifice of the left common carotid artery and myocutaneous flap reconstruction

Fig. 10.21
Cauterized adipose tissue. Axial CT image shows a nodular area with central hypoattenuation in the subcutaneous tissues of the right face (arrow), which represents biopsy-proven fibroadipose tissue

Fig. 10.22
Suture granuloma. AxialCT image after total laryngectomy shows a nodule with a central highattenuation focus (arrow)
Postoperative imaging is usually performed with contrast-enhanced CT and serves to identify clinically occult disease, which occurs in 7.5–28% of cases. Per NCCN guidelines, a baseline study is usually obtained within about 6 months of surgery for comparison with subsequent studies, which are usually obtained depending on symptoms, physical exam, and findings from previous imaging. During the early postoperative period, local hemorrhage and edema are common findings for all types of neck dissection. These changes usually resolve by 6 weeks after surgery and have a reticular appearance on CT. Over time, there may be persistence of edema and development of fibrosis, particularly if radiotherapy is administered. The degree of retropharyngeal edema can be exacerbated by resection of the internal jugular vein. A stable postoperative appearance may not be attained until 12–18 months after surgery.
Following selective and modified radical neck dissection, atrophy of the sternocleidomastoid and strap muscles is also common. In addition, removal of the adipose tissue and lymph nodes around the carotid sheath decreases the space between the sternocleidomastoid and internal jugular veins. The presence of scarring may accentuate the degree of asymmetry and effacement of the fat planes. Nonvisualization of the ipsilateral internal jugular vein occurs in about 20% of cases of selective neck dissection and may be attributable to thrombosis and should be reported. Following removal of the ipsilateral submandibular gland with level I dissection, the remaining contralateral submandibular gland should not be misinterpreted as a lesion itself. Radical neck dissection results in more conspicuous flattening of the neck contour and blurring of the tissue planes than modified radical neck dissection. There may also be reduced flow in the carotid artery due to surgical manipulation and radiation therapy. Rarely, the common carotid artery is sacrificed during extended neck dissection. Myocutaneous flaps are usually required to reconstruct large defects resulting from radical and extended neck dissection. Tissue flaps are also sometimes used to provide coverage of the external carotid artery after modified radical neck dissection.
Potential pitfalls in the interpretation of imaging after neck dissection has been performed are cauterized adipose tissue and suture granulomas, which can be mistaken for lymphadenopathy. Cauterized adipose tissue can appear as nodular foci of soft tissue attenuation, but often conserve some degree of fat attenuation centrally (Fig. 10.21). Suture granulomas can also appear as soft tissue nodules, but the occasional presence of hyperattenuation centrally associated with the suture is a help clue (Fig. 10.22). In either case, these conditions tend to remain static on serial imaging, as opposed to recurrent neoplasm.
Complications depend on the extent of neck dissection. Denervation injury results in high T2 signal and enhancement within the first few months and fatty atrophy and laxity on a chronic basis. The more frequently encountered sites for denervation injury after neck dissection include the trapezius muscle and the tongue (Fig. 10.23). Extensive neck dissection can potentially impair lymphatic drainage and lead to cervicofacial edema, which appears as diffuse swelling and fat stranding (Fig. 10.24) and can be exacerbated by radiation therapy. Leakage of chyle from the lymphatic system can result in lymphoceles, which typically appear as unilocular fluid collections with thin walls (Fig. 10.25). Infections can occur in the skin and subcutaneous tissue as cellulitis and abscesses (Fig. 10.26). In addition, osteomyelitis of the clavicle can result from lower central compartment or supraclavicular lymph node dissection (Fig. 10.27). This should not be confused with degenerative changes and effusions of the sternoclavicular joint due to altered biomechanics and neuropathic joint (Fig. 10.28).
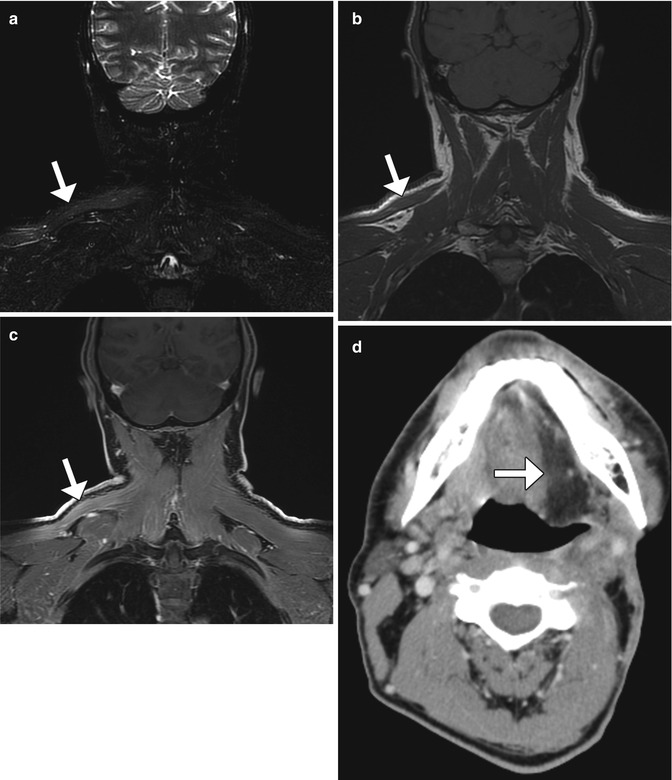
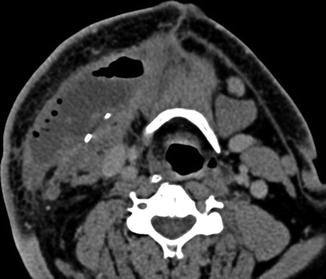
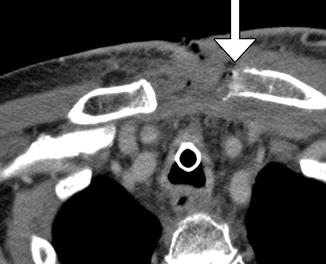


Fig. 10.23
Denervation related to neck dissection. Coronal STIR (a), T1-weighted (b), and post-contrast fat-suppressed T1-weighted (c) MR images show edema, and enhancement is an atrophic right trapezius muscle (arrow), ipsilateral to where neck dissection was performed. Axial CT image in a different patient (d) demonstrates fatty change in the left half of the tongue (arrow) after hypoglossal nerve sacrifice during neck dissection

Fig. 10.24
Postoperative lymphedema. Sagittal CT image shows diffuse swelling of the cervicofacial soft tissues, particularly the tongue and lips, as well as diffuse fat stranding

Fig. 10.25
Lymphocele. Axial CT image shows a large, unilocular fluid-attenuation collection (*) in the left neck that extends into the left chest subcutaneous tissues (Courtesy of John Wandtke, M.D.)

Fig. 10.26
Wound abscess. There is a large gas and fluid collection in the right neck surgical bed. There is also overlying subcutaneous fat stranding and skin thickening

Fig. 10.27
Osteomyelitis. Axial CT image shows an open wound and sinus tract with regional subcutaneous fat stranding and erosion of the left medial clavicle (arrow)

Fig. 10.28
Neuropathic joint. Coronal CT image (a) shows radical right neck dissection and flap reconstruction. Axial CT image (b) shows right proximal clavicle degenerative changes ipsilateral to the neck dissection
10.3 Parotidectomy
10.3.1 Discussion
Parotidectomy is most commonly performed for primary salivary neoplasm resection, but is also performed for oncologic management of skin cancers. Several types of parotidectomy can be implemented, including superficial parotidectomy and total parotidectomy with or without facial nerve preservation, depending on the type, size, and location of the tumor (Figs. 10.29, 10.30, and 10.31). The defects created by more extensive resections can be reconstructed using tissue flaps or synthetic materials. Furthermore, when the facial nerve is compromised, eyelid weights are often used to aid eye closure.
In general, complications and expected consequences related to parotidectomy may include cosmetic deformity, facial nerve deficits, sialocele, wound infection, hematoma, and tumor recurrence. In particular, recurrence of parotid pleomorphic adenoma has an incidence of 1–5% and most commonly occurs within the first 10 years following surgical resection. Recurrent lesions have fairly characteristic imaging features. On T2-weighted MRI, the majority of recurrent tumors have very high signal intensity due to myxoid contents. The presence of multiple subcentimeter nodules is a strong indicator of recurrence and is observed in about two-thirds of cases. This feature results in a “bunch of grapes” appearance (Fig. 10.32). Recurrent pleomorphic adenomas are sometimes located in the subcutaneous tissues or adjacent neck spaces perhaps due to spillage during surgery. The enhancement pattern is variable, depending upon the extent of cystic components, fibrosis, and necrosis.
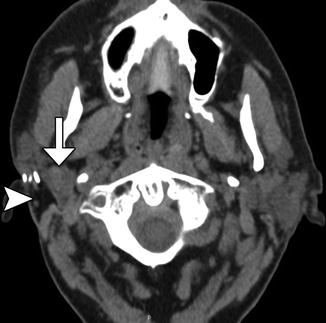

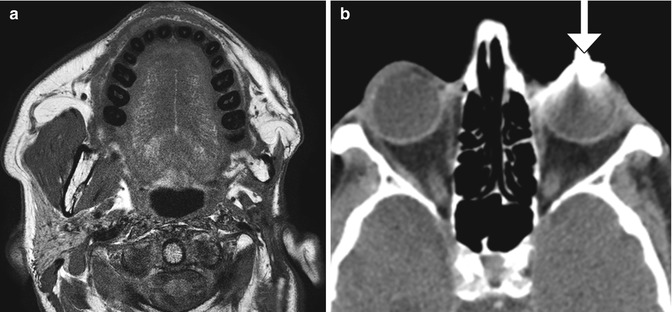
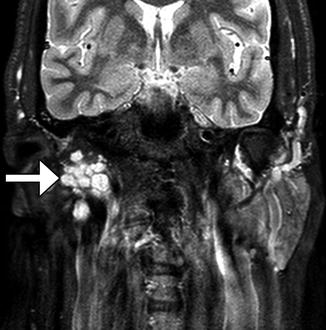

Fig. 10.29
Superficial parotidectomy with graft reconstruction. Axial CT image shows fat graft occupying the expected location of the right superficial parotid lobe (arrowhead). The deep lobe of the right parotid gland remains intact (arrow)

Fig. 10.30
Total parotidectomy. Axial T2-weighted MRI shows the absence of the left parotid gland, resulting in concavity of the overlying skin. The facial nerve could be spared along with the retromandibular vein, and the contralateral normal parotid gland is intact

Fig. 10.31
Total parotidectomy with facial nerve sacrifice. Axial T1-weighted MRI (a) shows the absence of the left parotid gland and atrophy of the left facial muscles. Partial resection of the left masticator muscles and mandibular ramus was also performed. The axial CT image (b) shows a left eyelid weight (arrow), with considerable metal streak artifact

Fig. 10.32
Recurrent parotid pleomorphic adenoma. Coronal STIR MRI demonstrates a cluster of nodules with a “bunch of grapes” appearance (arrow)
10.4 Salivary Duct Stenting and Endoscopic Stone Removal
10.4.1 Discussion
Salivary duct stones can be managed by sialendoscopic extraction. Sometimes, plastic stents are inserted after stone removal in order to reduce the risk of subsequent stenosis (Fig. 10.33). These appear as tubular hyperattenuating structures on CT and should not be misinterpreted as residual sialolithiasis. Occasionally, stone extraction can be complicated by sialocele or even cutaneous fistula formation due to the friability of the inflamed tissues in the setting of acute sialadenitis and sialodacryoadenitis. In such cases, imaging can be performed to assess for the extent of associated fluid collections and sinus tracts (Fig. 10.34).
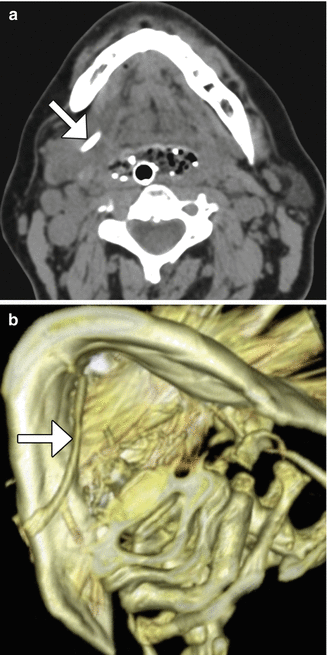
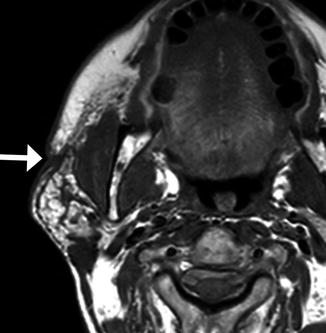

Fig. 10.33
Submandibular duct stent. Axial (a) and 3D CT (b) images show a hyperattenuating stent that passes through the right submandibular duct (arrows)

Fig. 10.34
Parotid cutaneous fistula after endoscopic stone extraction. Axial T1-weighted MRI shows a right face skin defect and sinus tract (arrow) extending to the underlying parotid gland
10.5 Facial Reanimation
10.5.1 Discussion
Facial reanimation can be performed for treating the effects of chronic facial nerve paralysis. This can be accomplished with techniques, such as functioning free muscle transfer or temporalis muscle transposition and suspension combined with suborbicularis oculi fat (SOOF) lift. Overall, these techniques successfully restore smiles and provide improvement in mouth function in most patients.
Functioning free gracilis microneurovascular muscle transfer is a form of dynamic facial reanimation that can help restore facial tone and movement. The free muscle flap is buried in the subcutaneous tissues of the face extending from the temporal fossa to the oral commissure region. CT and MRI can demonstrate the intact muscle fibers in the healthy grafts (Fig. 10.35). In addition, Doppler ultrasound is useful for evaluating the patency of the feeding artery and draining vein. Transfer of compound flaps containing muscle and other tissue, such as the skin, can be performed for cases of complex facial paralysis that involve skin or soft tissue deficits after tumor excision. Alternatively, tensor fascia lata and AlloDerm grafts can be used and also appear as soft tissue bands on imaging, but these do not offer dynamic facial animation (Figs. 10.36 and 10.37).
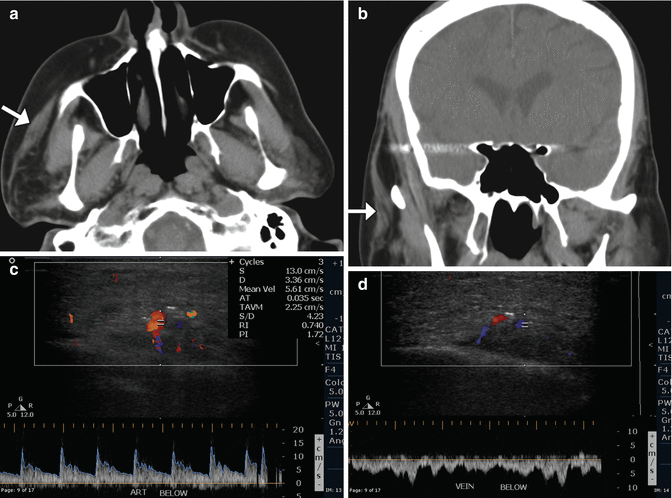
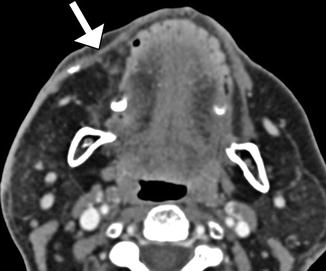
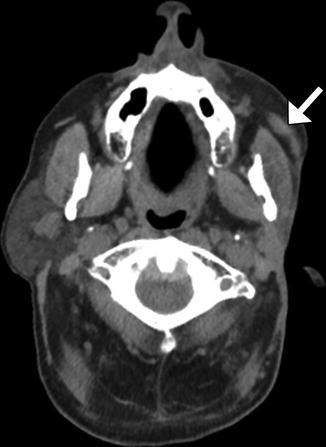

Fig. 10.35
Free gracilis muscle transfer. The patient had right facial paralysis after right cerebellopontine angle schwannoma resection. Axial (a) and coronal (b) CT images demonstrate the grafted muscle (arrows) within the right face subcutaneous tissues. Doppler ultrasound images of the graft artery (c) and vein (d) display normal waveforms

Fig. 10.36
Tensor fascia lata graft. Axial CT image shows the band-like graft positioned in the right face subcutaneous tissues, inserting into the oral commissure (arrow)

Fig. 10.37
AlloDerm graft. The patient is status post total left parotidectomy with facial nerve sacrifice. Axial CT image shows the soft tissue attenuation sling (arrow) in the left check subcutaneous tissues
Temporoparietal fascia and temporalis muscle transposition and suspension procedures consist of detaching and repositioning the flap approximately 180° inferiorly toward the oral commissure and/or nasolabial folds via a tunnel through subcutaneous tissues (Figs. 10.38 and 10.39). The tissues superficial to the plane of dissection can be translated superomedially and sutured to the fascia of the temporalis muscle. If necessary, the procedure can be augmented using Silastic prostheses to fill the defect. Alternatively, the muscle can be extended using polytetrafluoroethylene.
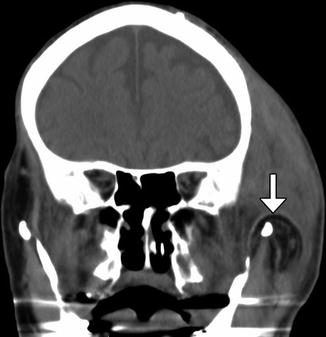
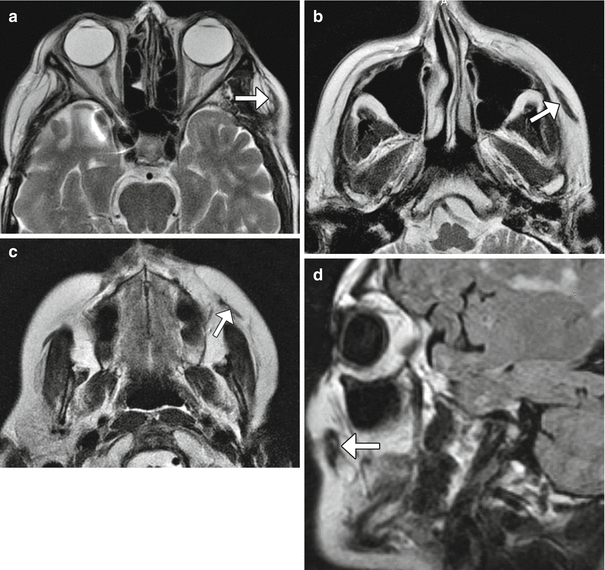

Fig. 10.38
Temporoparietal fascia and muscle flap. The patient has a history of left facial paralysis. Coronal CT image shows the flap swung inferiorly over the zygomatic arch (arrow). There is considerable soft tissue swelling at the surgical site

Fig. 10.39
Temporalis muscle transposition and suborbicularis oculi fat (SOOF) lift. The patient had left facial paralysis status post parotidectomy and facial nerve resection for adenoid cystic carcinoma. Serial axial T2-weighted MR images from superior to inferior (a–c) and a sagittal T2-weighted FLAIR image (d) show the left temporalis (arrows) turned inferomedially toward the mouth. The suborbicularis oculi fat pad has also been raised
The suborbicularis oculi fat (SOOF) lift involves superior mobilization of midface structures, which are fastened to the orbital rim using a variety of approaches (Fig. 10.40). Often, the intraorbital fat pads are also released and sutured to the SOOF.
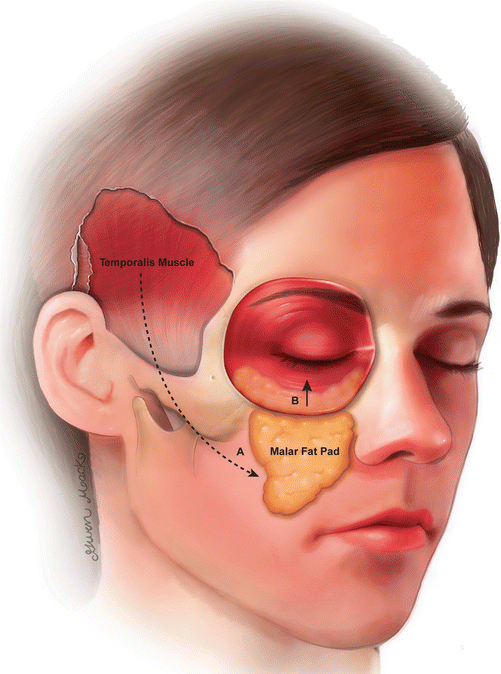

Fig. 10.40
Schematic of the temporalis transposition technique. In the temporalis transposition (A), the temporalis muscle is detached from the calvarium and brought inferomedially over the zygoma toward the oral commissure and nasolabial folds. In the SOOF lift (B), the suborbicularis oculi fat pad is repositioned superiorly
10.6 Oral Cavity Tumor Resection and Reconstruction
10.6.1 Discussion
Depending on the stage of oral tongue malignancies, such as squamous cell carcinomas, variable degrees of glossectomy may be performed, ranging from partial, subtotal, or total, with or without floor of the mouth resection, mandibulectomy, and laryngectomy (Figs. 10.41, 10.42, 10.43, 10.44, and 10.45). Of note, composite tumor resection consisting of glossectomy, mandibulectomy, and neck dissection known as “Commando,” an acronym for combined mandibulectomy and neck dissection operation, can be performed for advanced cancers of the oral cavity. Furthermore, the submandibular gland may be removed with rerouting of the duct as part of the approach or as part of the combined suprahyoid neck dissection. Alternatively, the submandibular gland may be the main target of surgery when it is involved by primary salivary gland neoplasms. There are a variety of options for reconstructing surgical defects in the oral cavity region, including myocutaneous flaps, such as single or double bilobed radial forearm flaps, FAMM flaps, submental island flaps, and acellular dermal matrix, or a combination of these.
The role of imaging after glossectomy is to evaluate complications, such as infection, sialocele, and tumor recurrence (Figs. 10.46, 10.47, and 10.48). Of note, one must be particularly vigilant for the presence of perineural tumor spread on imaging before and after surgery, especially following resection of salivary gland malignancies, which is often along the maxillary division branches of the trigeminal nerve for oral cavity tumors. Furthermore, since radiation often accompanies surgical treatment of oral cancers, the mandible is at risk for osteonecrosis. This complication tends to occur at least 1 year after radiation therapy and appears as areas of cortical irregularity and lucency (Fig. 10.49). There can be superimposed infection and pathological fracture.

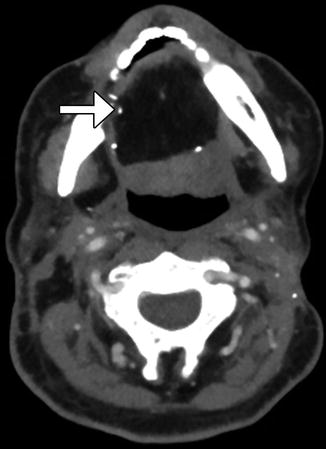
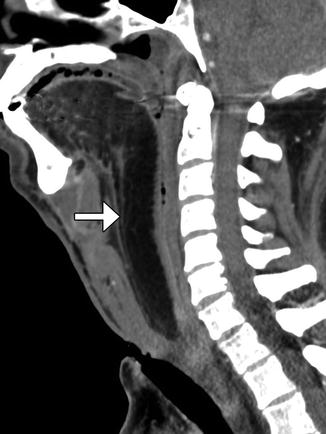
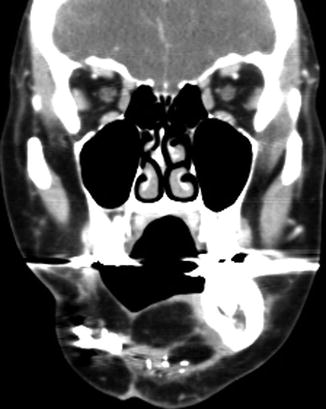
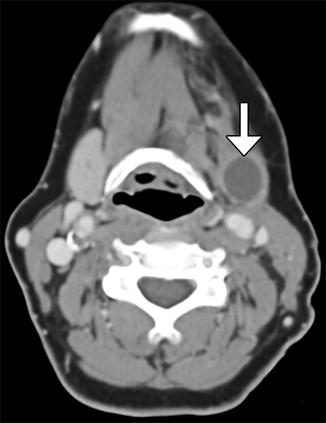
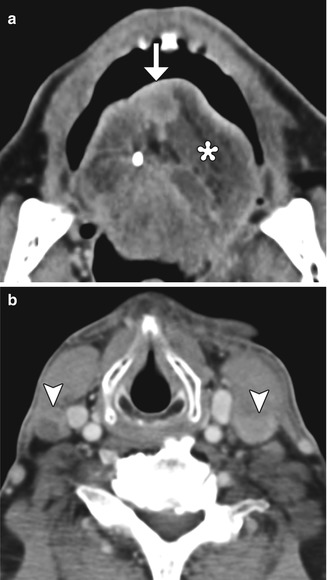
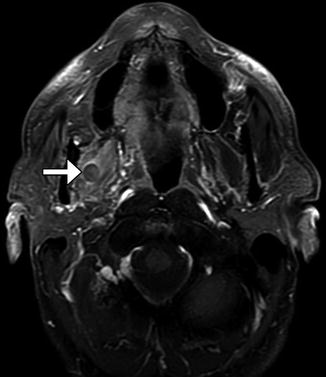
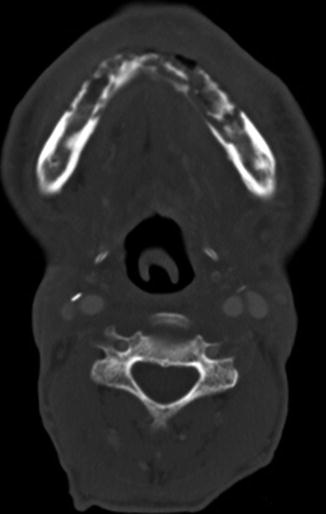

Fig. 10.41
Partial hemiglossectomy with primary closure. Coronal fat-suppressed post-contrast T1-weighted MRI shows a defect in the left lateral tongue (arrow), without graft reconstruction resulting in asymmetric prominence of the normal right side of the tongue

Fig. 10.42
Subtotal glossectomy. The patient had a history of squamous cell carcinoma of the tongue. Axial CT image shows that the majority of the oral tongue has been resected and reconstructed using a myocutaneous graft (arrow). Surgical clips are present along the margins of the graft

Fig. 10.43
Total glossectomy and laryngectomy. The patient had a history of chemoradiation for stage IV squamous cell carcinoma of the base of the tongue. Subsequently, total laryngectomy and total glossectomy with myocutaneous flap reconstruction were performed. Sagittal CT image demonstrates complete absence of the native tongue with placement of a myocutaneous flap with predominantly fat attenuation components (arrow). The flap provides near-anatomic contours for the reconstructed tongue

Fig. 10.44
Commando. Coronal CT image shows glossectomy, and right hemimandibulectomy with flap reconstruction. Neck dissection, which is not shown, was also performed

Fig. 10.45
Floor of mouth resection with marginal mandibulectomy. Sagittal CT image shows extensive resection of the floor of mouth contents along with the gingiva and alveolar portions of the mandible. The defect has been reconstructed using a myocutaneous flap

Fig. 10.46
Sialocele after floor of mouth resection and submandibular duct rerouting. Axial CT image shows a well-defined fluid collection in the right submandibular space (arrow)

Fig. 10.47
Locoregional tumor recurrence. Axial CT images (a, b) show recurrent tumor (arrow) at the glossectomy site (*) as well as bilateral lymphadenopathy (arrowheads) from metastatic squamous cell carcinoma

Fig. 10.48
Perineural tumor. Axial fat-suppressed post-contrast T1-weighted MRI shows marked expansion of a branch of the right maxillary division of the trigeminal nerve that represents perineural tumor (arrow) that remained after resection of a submandibular gland adenoid cystic carcinoma at another institution

Fig. 10.49
Mandibular osteonecrosis. Axial CT image shows extensive irregular lucency on the mandible after radiation and floor of mouth tumor resection
10.7 Tonsillectomy and Adenoidectomy
10.7.1 Discussion
Tonsillectomy and adenoidectomy are two of the most commonly performed pediatric surgical procedures. The main indications for tonsillectomy and adenoidectomy include adenotonsillar hyperplasia with obstructive sleep apnea, failure to thrive, or abnormal dentofacial growth; malignant neoplasms; and adenotonsillar hyperplasia with upper airway obstruction, dysphagia, or speech impairment and halitosis. Furthermore, otitis media and recurrent or chronic rhinosinusitis or adenoiditis are indications for adenoidectomy, but not tonsillectomy, while recurrent or chronic pharyngotonsillitis, peritonsillar abscess, and streptococcal carriage are indications for tonsillectomy, but not adenoidectomy. Frequently, the appearance on postoperative imaging is that of asymmetric absence of Waldeyer ring tissue, whereby the residual normal tissue can hypertrophy and should not be mistaken for a lesion (Fig. 10.50). Sometimes, the trace amounts of residual Waldeyer ring tissues can regrow over the course of years after tonsillectomy/adenoidectomy, such that the effects of surgery are not noticeable. As in many other parts of the head and neck, when more extensive surgeries are performed for tumor resection, the resulting defects may be reconstructed using soft tissue flaps (Fig. 10.51).
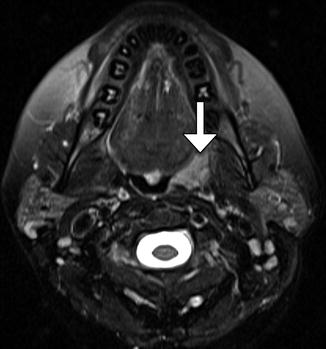
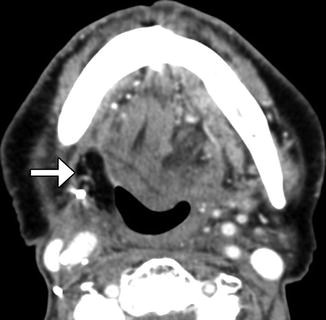

Fig. 10.50
Tonsillectomy. Axial fat-suppressed T2-weighted MRI shows the absence of the right lingual tonsil and a remaining hypertrophied left anterior palatine tonsil (arrow). The postoperative changes are otherwise virtually imperceptible

Fig. 10.51
Tonsillectomy with flap reconstruction. Axial CT images show flap reconstruction of the right tonsillectomy defect (arrow), after resection of an invasive squamous cell carcinoma. There is nevertheless a relative paucity of tissue on the right side
Among patients who underwent tonsillectomy for obstructive apnea, cine MRI is a useful modality for evaluating anatomy and function when there are recurrent symptoms. Potential causes include glossoptosis, hypopharyngeal collapse, recurrent and enlarged adenoids and lingual tonsils, and macroglossia. If lingual tonsils were greater than 10 mm in diameter and abutted both the posterior border of the tongue and the posterior pharyngeal wall, they can be considered markedly enlarged (Fig. 10.52). On the other hand, patients can develop velopharyngeal insufficiency following excessive removal of adenoid tissues. This can manifest as a gap between the pharynx and soft palate on cine MRI, which can be treated via palatoplasty or pharyngeal augmentation with substances, such as hydroxyapatite filler (Fig. 10.53).

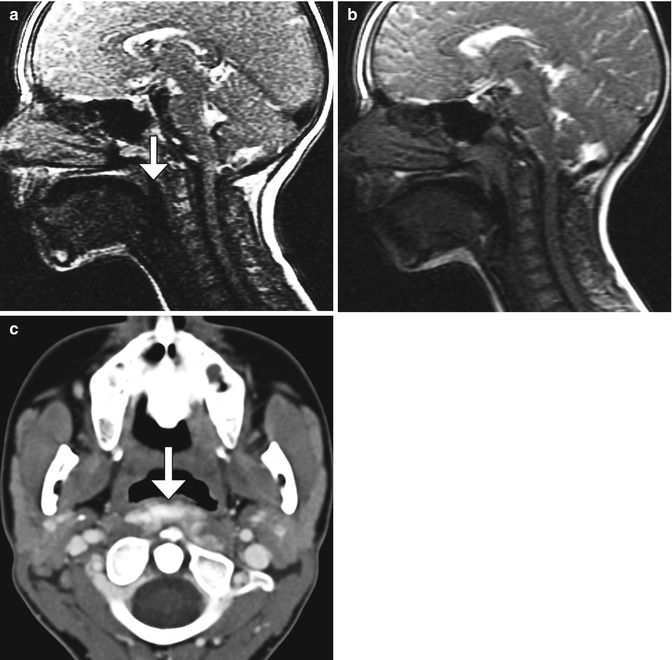

Fig. 10.52
Recurrent enlargement of adenoids and tonsils. Cine MRI in a child with obstructive apnea previously treated with adenotonsillectomy shows enlarged adenoid and lingual tonsils associated with airway narrowing (encircled)

Fig. 10.53
Velopharyngeal insufficiency after adenoidectomy. The patient underwent a Furlow palatoplasty to repair a submucosal cleft with marked improvement but persistent velopharyngeal insufficiency with fatigue at the end of the day. Posterior pharyngeal wall pharyngoplasty with calcium hydroxyapatite filler injection augmentation was then performed. Sagittal cine MR image (a) after adenoidectomy and palatoplasty shows velopharyngeal gap that persists throughout the cycle (arrow). Sagittal MR image (b) obtained after adenoid augmentation shows increased bulk of the adenoids with no residual velopharyngeal gap. Axial CT image (c) in a different patient shows the high attenuation Radiesse within the retropharyngeal space at the level of the oro- and nasopharynx (arrow)
Among patients who underwent tonsillectomy/adenoidectomy for neoplasm, 18FDG-PET/CT is useful for evaluating recurrent tumor. However, infection at the site of surgery can manifest as focal hypermetabolism (Fig. 10.54). Noninfectious inflammation and granulation tissue at the surgical site can also yield false-positive results on 18FDG-PET/CT. However, as opposed to tumor recurrence, activity on 18FDG-PET/CT should decrease over time with infection and inflammation.
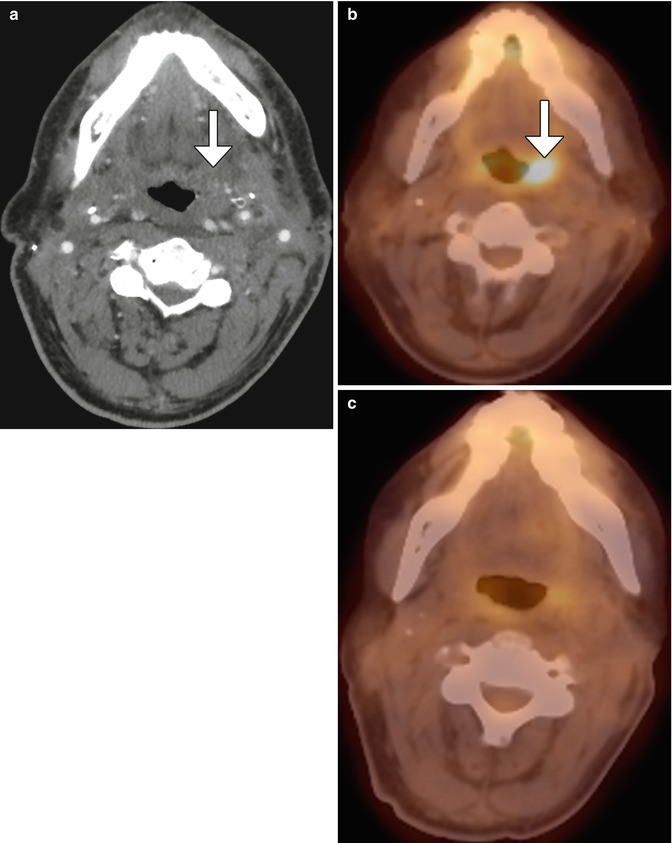

Fig. 10.54
Postoperative infection mimicking tumor recurrence. Contrast-enhanced CT (a) shows asymmetric edema of the pharyngeal mucosal and parapharyngeal spaces (arrow), but no distinct mass. 18FDG-PET/CT (b) obtained soon after shows focal hypermetabolism in the left oropharyngeal surgical bed (arrow). The lesion proved to be fungal pharyngitis, and follow-up 18FDG- PET/CT (c) obtained 6 months later showed resolution of the lesion
10.8 Transoral Robotic Surgery
10.8.1 Discussion
Transoral robotic surgery (TORS) is a minimally invasive technique that involves the use of endoscopic visualization and dexterous robotic arms and has been mainly implemented for resecting T1 and T2 squamous cell carcinomas of the oropharynx, although various other applications have been explored. TORS offers a high rate of preserved postoperative swallowing function, but low incidence of complications. The postoperative imaging findings to TORS generally differ from those related to open surgery. Tongue base tumor TORS resection typically includes approximately the half of the tongue base on the side of the tumor, with dissection to the circumvallate papillae and glossotonsillar sulcus. Consequently, during the first weeks to months after surgery, retraction of the tongue base bed is apparent on postoperative imaging (Fig. 10.55), without evidence of solid enhancement. A radical tonsillectomy using a TORS approach involves the tonsil, anterior and posterior tonsillar pillars, portions of the soft palate, tongue base, to encompass the superior constrictor muscle as the depth of resection and the posterior pharyngeal wall are resected. Imaging during the first several postoperative weeks typically demonstrates distortion of the fat planes around the medial pterygoid muscle and retropharyngeal edema (Fig. 10.56), which can result from retraction or thermal injury during the surgery. Over the ensuing months, scar tissue formation leads to gradual retraction of the lateral oropharyngeal wall, with “tilting” of the soft palate toward the surgical bed.
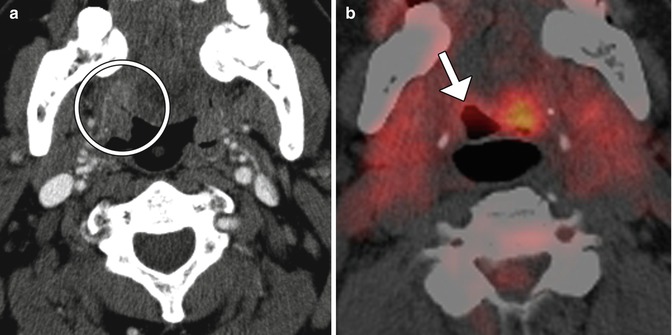






Fig. 10.55
TORS base of tongue tumor resection. Preoperative axial CT image (a) shows a right oropharyngeal tumor (encircled). Postoperative axial 18FDG-PET/CT image (b) shows retraction of the right tongue base surgical bed (arrow) without evidence of tumor, but residual normal hypermetabolic left lingual tissue, which should not be misinterpreted as tumor

Fig. 10.56
TORS lateral oropharyngectomy. Preoperative axial CT image (a) shows a right palatine tonsil squamous cell carcinoma (arrow). Postoperative CT image (b) shows interval resection of the tumor and edema in the region of the surgical bed, with extension into the retropharyngeal space (encircled)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree