1. Describe the basics of radionuclide imaging in infection and inflammation.
2. Compare pros and cons of imaging modalities in selected indications within the domain of infectious and inflammatory diseases.
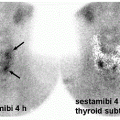
abdominal infection, especially if 99mTc-HMPAO is used, delayed images in suspected bone or prosthetic joint infection, or completely refraining from using labeled WBC if infection in the spine is suggested (5).
Table 9.1 OVERVIEW OF SELECTED COMMON INDICATIONS WITH POTENTIAL DIAGNOSTIC VALUE OF RADIONUCLIDE MODALITIES IN INFECTIOUS AND INFLAMMATORY DISEASES—NUMBERS ARE FURTHER EXPLAINED AND QUALIFIED IN THE TEXT | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
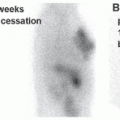
in suspected inflammatory disease should be performed before or shortly after treatment is instituted, and otherwise discontinuation should be considered (17).
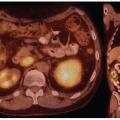
studies have found direct clinical impact of FDG-PET/CT on patient management such as changes in treatment in 47% to 74% of cases (35,36,37), and in one series relapse rates and mortality was similar in patients with uncomplicated bacteremia compared to a group with bacteremia with high risk of metastatic spread, but negative echocardiography and FDG-PET/CT who were treated with antibiotics for the same duration (40).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree