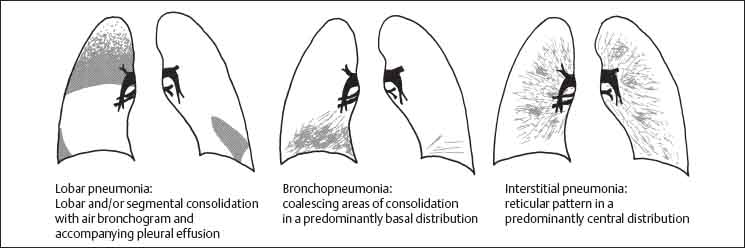
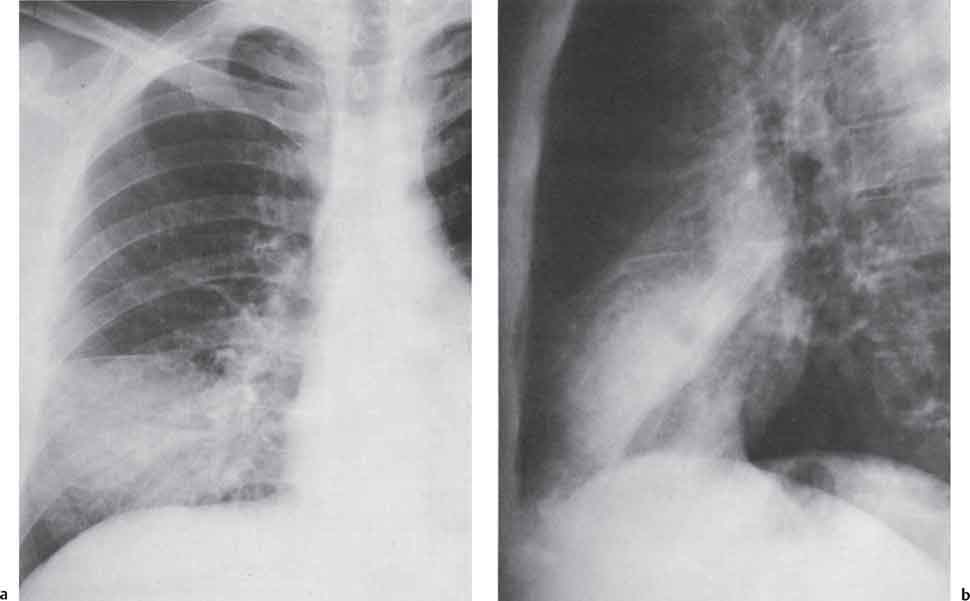
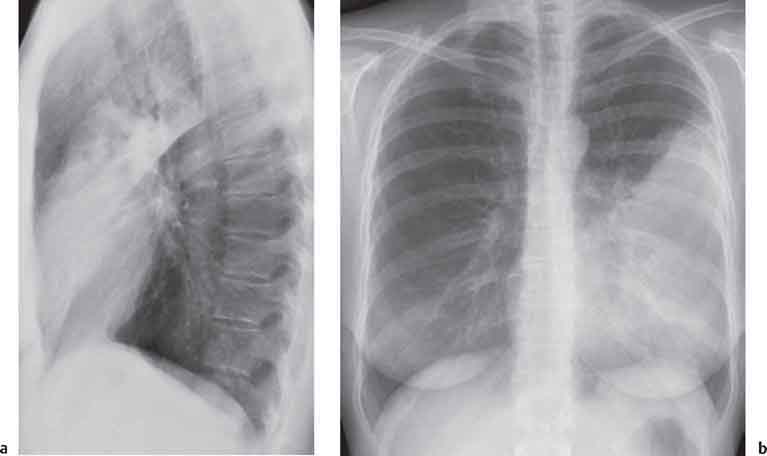
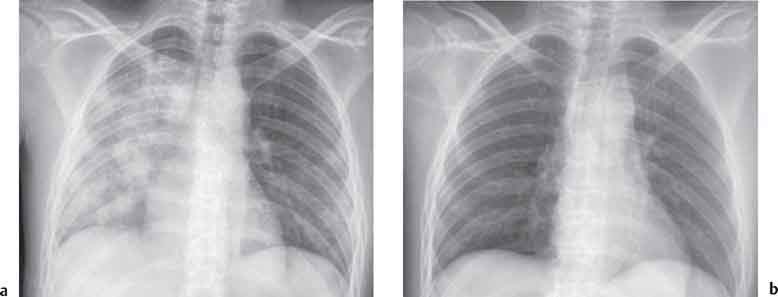
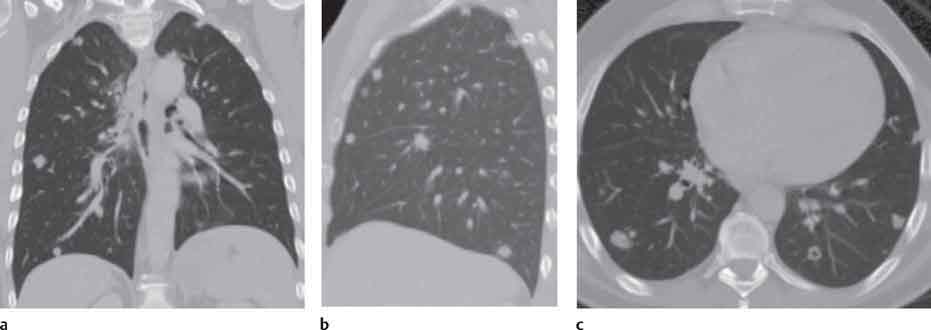
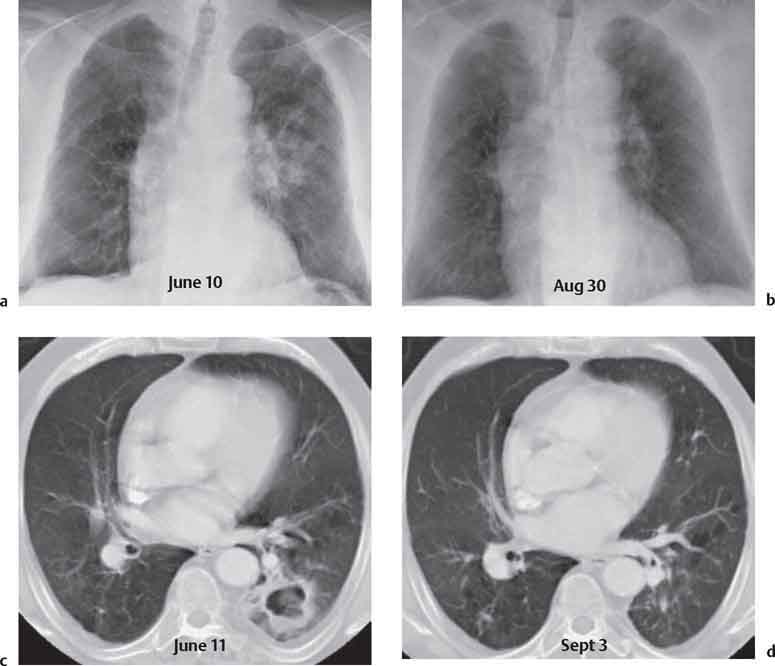
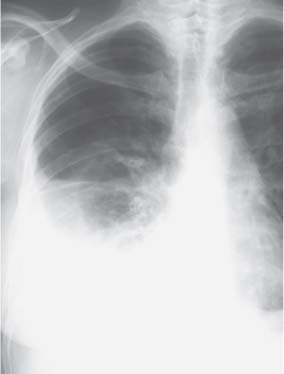
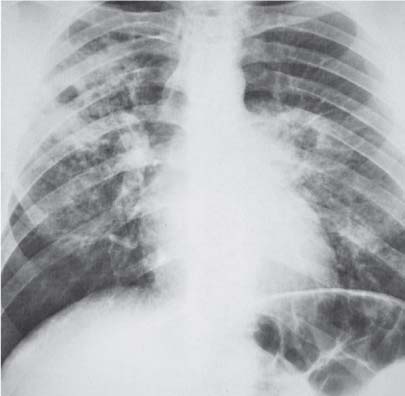
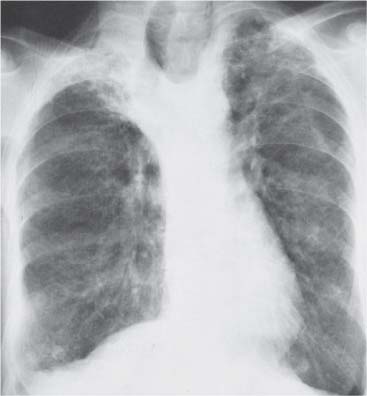
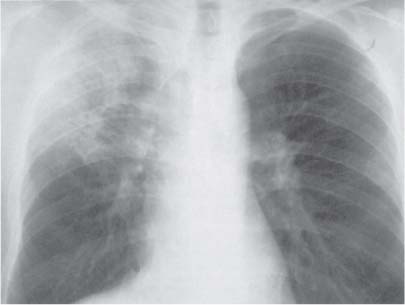
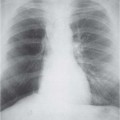
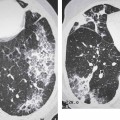
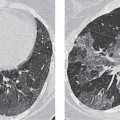
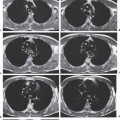
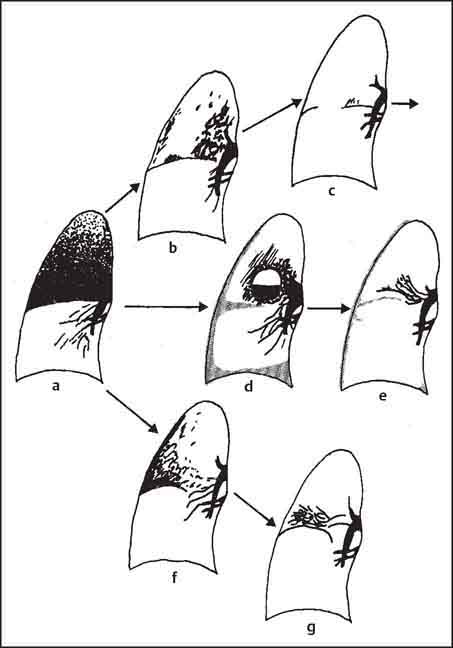
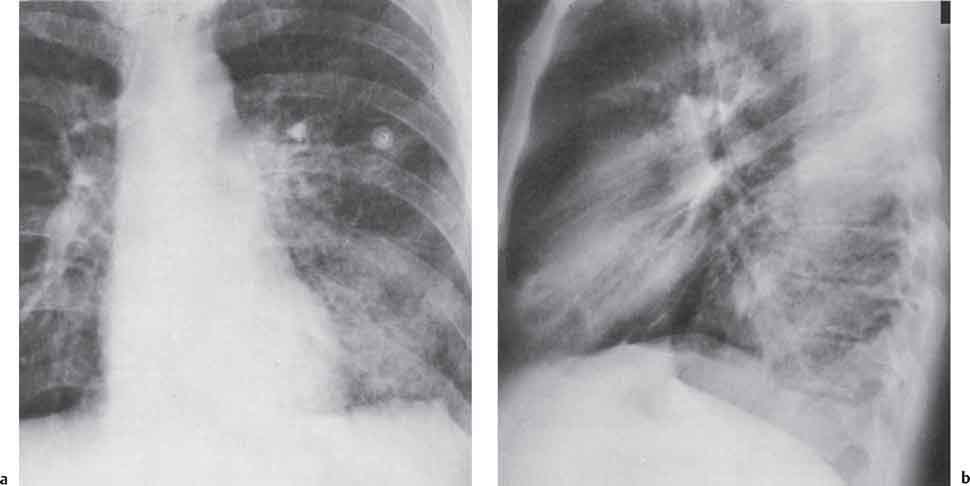
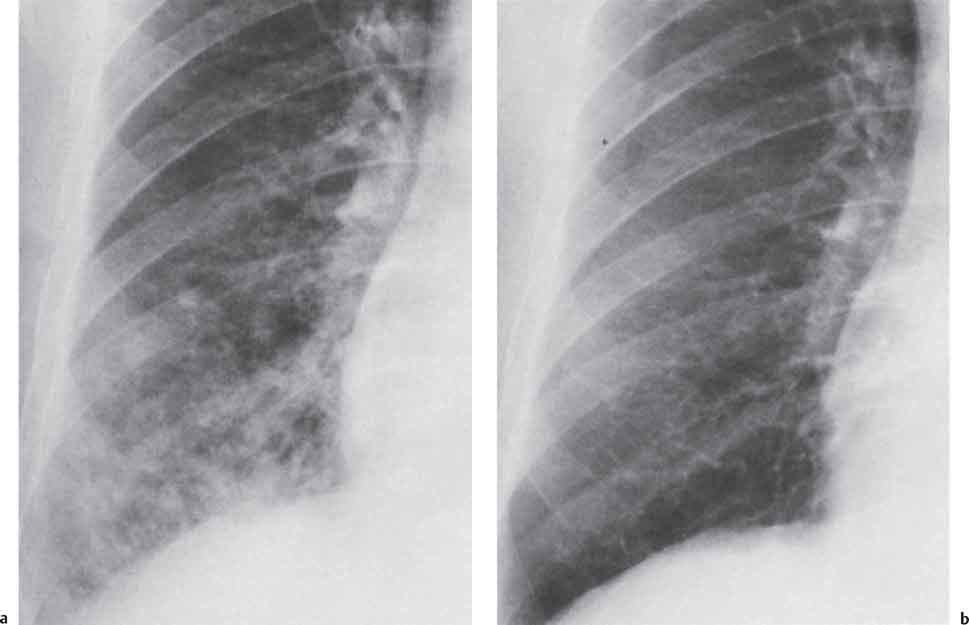
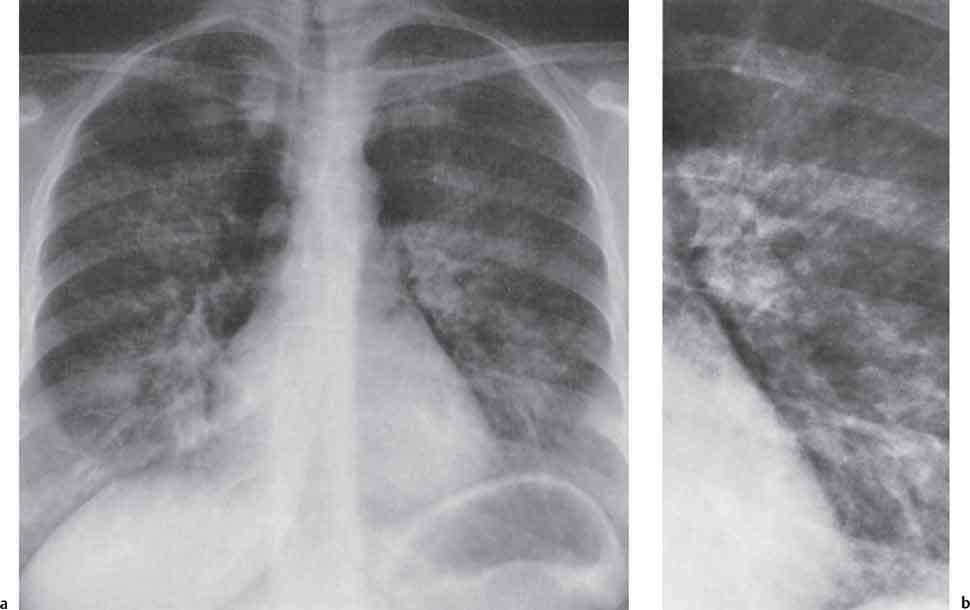
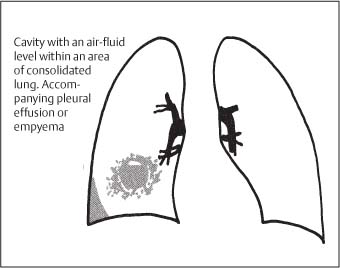
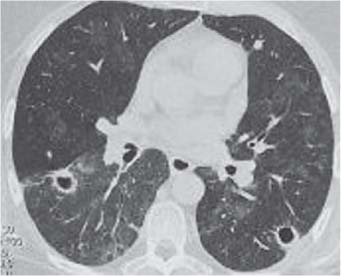
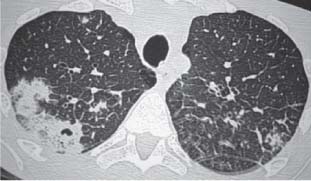
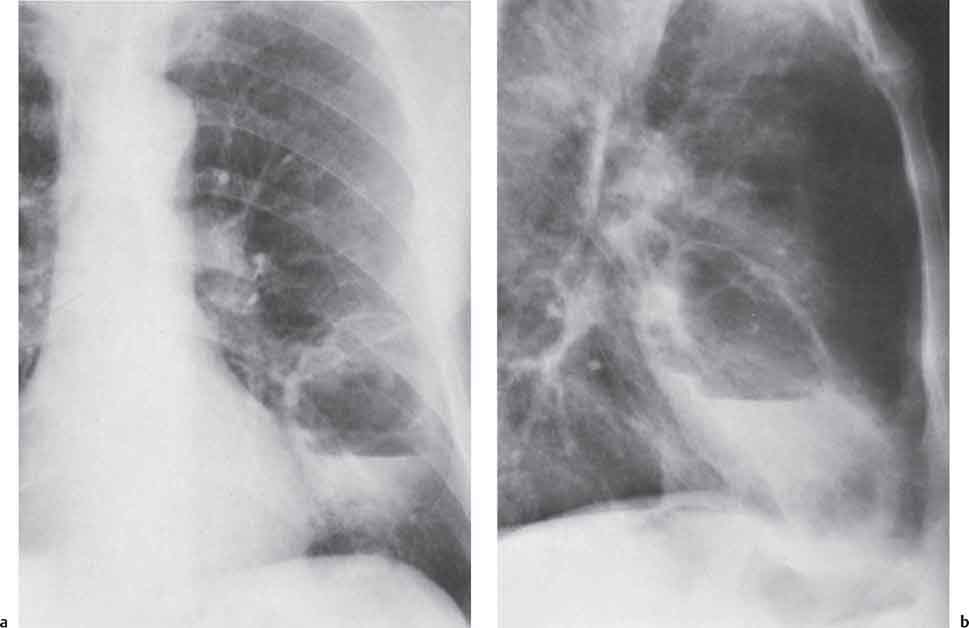
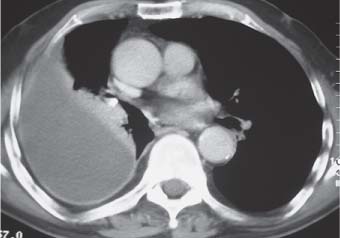
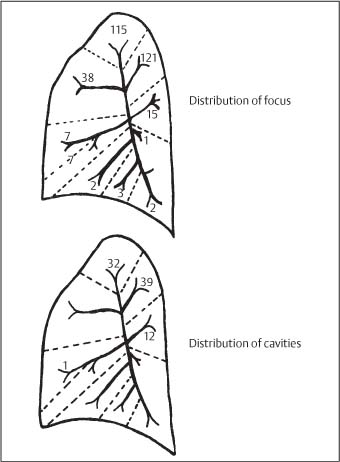
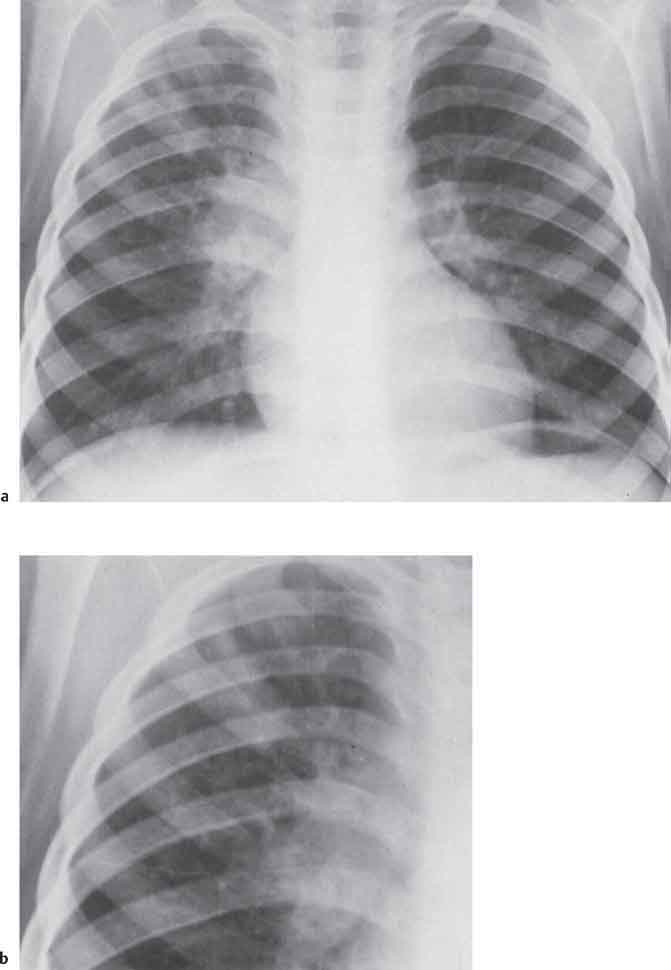
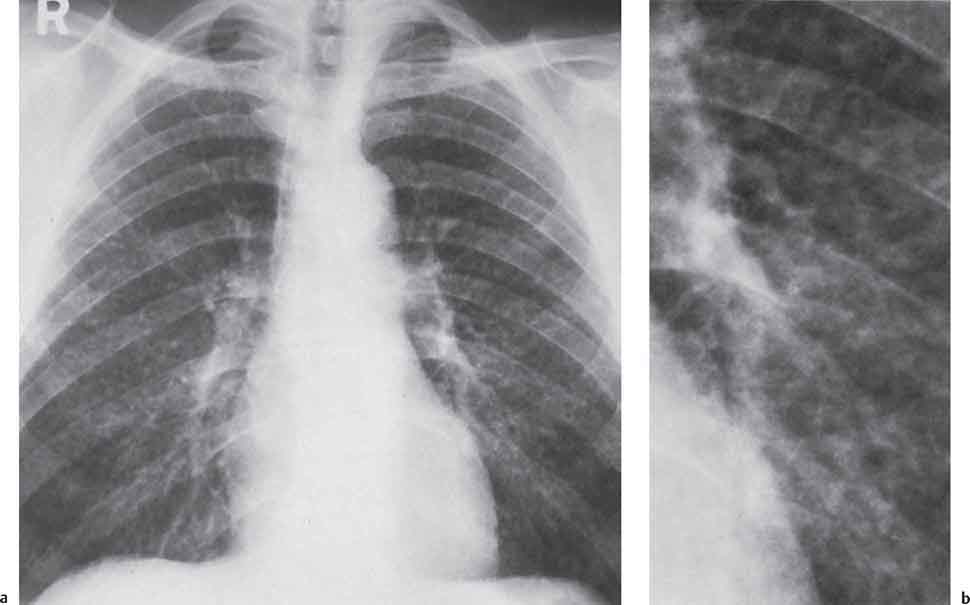
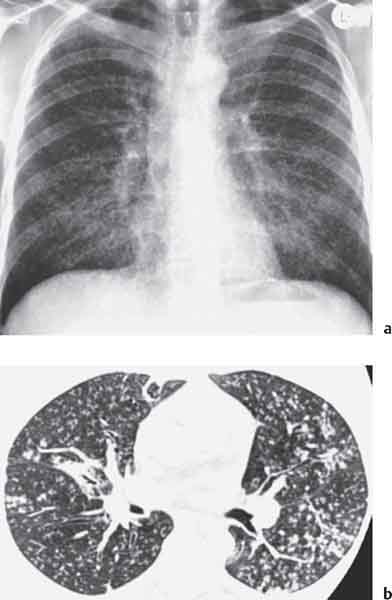
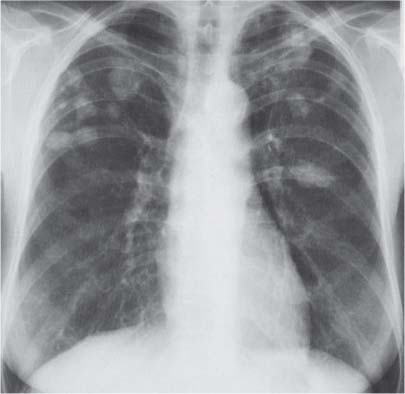
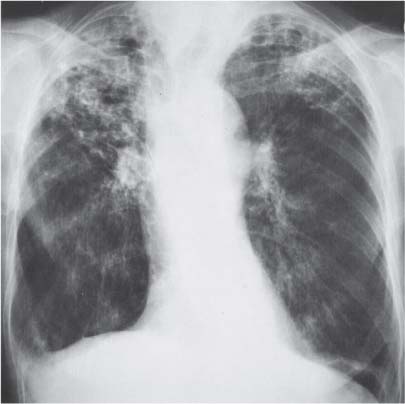
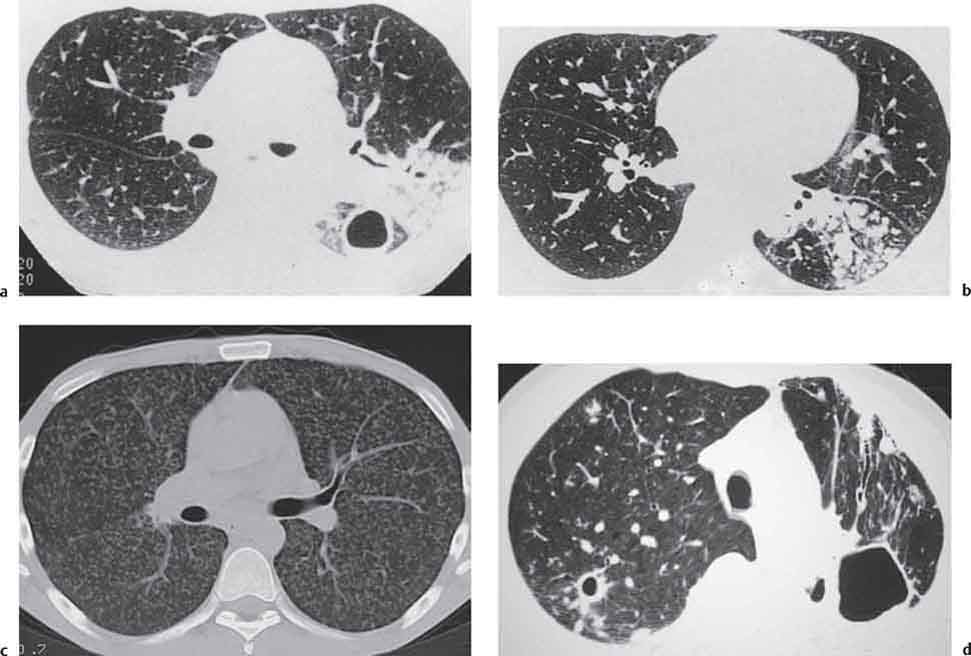
3 Infection and Inflammatory Disorders Pneumonia is an infectious pulmonary process that may be caused by bacteria, mycoplasma, viruses, and other microorganisms. It is characterized by inflammatory exudate in both the alveoli and interstitium. Determination of the causative organism by sputum bacteriology or immunoserology may be helpful in initiation of therapy. Some textbooks classify pneumonias according to the causative organism (Murray 2000). However, given the similarity of the clinical manifestations and radiographic findings produced by different organisms, we shall focus our discussion on their morphologic features. Fig. 3.1 Possible evolution of right upper lobar pneumonia. a, b, c Complete resolution. a, d, e Abscess formation with subsequent scarring and pleural thickening. a, f, g Pneumonia progressing to bronchiectasis (from Bohlig). Morphologic classification distinguishes between lobar pneumonia, bronchopneumonia, and interstitial pneumonia. This classification depends on the nature and extent of inflammatory exudate in the alveoli and the degree of interstitial infiltration. Lobar pneumonia is an alveolar or airspace consolidation in which the causative organisms are inhaled. On reaching the alveoli, they proliferate and spread rapidly through the pores of Kohn across segmental boundaries until the entire lobe is involved. In lobar pneumonia there is relative sparing of the bronchi and interstitium. The classic progression of lobar pneumonia through the stages of congestion (intra-alveolar edema), red hepatization (erythrocyte exudation), yellow hepatization (leukocyte exudation), gray hepatization (fibrinous change), and eventual lysis is rarely seen today. Effective antibiotic therapy also has led to a marked decrease in the incidence of lobar pneumonia. Bronchopneumonia is acquired by inhalation or, less commonly, by hematogenous spread of the causative organism. On reaching the terminal and respiratory bronchioles, the organisms precipitate an inflammatory reaction which then spreads to adjacent alveoli. Further extension through the pores of Kohn results in involvement of the entire secondary lobule. Bronchopneumonia tends to be multifocal and patchy in distribution, the infiltrated lobules being interspersed between areas of normally aerated lung. The most common causative organisms are Staphylococcus aureus, Haemophilus influenzae, Pseudomonas species, and anaerobes (Webb et al. 1992). Fig. 3.2 Radiographic patterns of pneumonia. Inflammatory infiltration of the connective tissue framework of the lung is characteristic. Causative organisms include Mycoplasma, Rickettsia, and viruses. On reaching the bronchial wall via the airways, they destroy the ciliated epithelium, with resulting edema and lymphocytic infiltration of the bronchial mucosa. There is subsequent spread of the inflammatory process to the interlobular septa. There also is lymphocytic infiltration of the peribronchial alveoli and this appears similar to lobular pneumonia. Clinical manifestations include pyrexia, pleuritic chest pain, and cough productive of serous, purulent, or blood-stained sputum. Auscultatory findings of fine bubbling rales correlate with radiographic change in only 40% of cases. Leucocytosis with left shift and elevated antibody titers also may be present. Bacteriological diagnosis may be possible from sputum analysis with Gram stain and culture; serology may yield the diagnosis in atypical bacterial and viral pneumonia. The pathomorphologic classification of pneumonias may also be used to characterize their radiographic features, although differentiation is not always possible (Fig. 3.2). Lobar Pneumonia In classic lobar or segmental pneumonia, the radiographic features are characteristic with homogeneous opacification of the involved lobes or segments (Figs. 3.3–3.5). Segments often are incompletely opacified, and this makes their location difficult to determine except when the shadowing extends to a well-defined pleural/fissural margin. Patent bronchi within homogeneous consolidation appear as linear branching lucencies or, when seen end on, as rounded lucencies (air bronchogram). The volume of the affected segments may be diminished because of inflammatory narrowing of the airways and decreased surfactant production. Classic lobar pneumonia, caused by Streptococcus pneumoniae in 95% of cases, is extremely rare today. Segmental opacification, however, is quite common and very often represents confluent multifocal consolidation. Bronchopneumonia In bronchopneumonia, the radiologic pattern is that of multiple, ill-defined, confluent, nodular opacities, which represent multiple secondary lobules filled with inflammatory exudate (Figs. 3.6, 3.7). The nonhomogeneous pattern of ventilated and consolidated lobules results in a sponge-like pattern. Focal air trapping within secondary lobules due to check-valve obstruction of bronchioles may also contribute to this appearance. Poor aeration of the infected lung may lead to basal opaque bands similar to discoid atelectasis. Interstitial Pneumonia Inflammatory infiltration of the bronchial wall and interlobular septa leads to formation of linear and reticular opacities most marked in the perihilar lung. Simultaneously, focally confluent shadows are found which represent inflammatory exudate in the peribronchiolar alveoli (Fig. 3.8). Fig. 3.3a, b Pneumonia in the lateral segment of the right middle lobe: homogeneous consolidation with a sharp boundary anterosuperiorly at the minor fissure (a) and a well-defined posterior boundary at the major fissure (b). Patient presented clinically with pyrexia, productive cough, and leukocytosis. Fig. 3.4a, b Left lower lobe pneumonia. Patchy consolidation is seen to obliterate the posterior aspect of the left hemidiaphragm in the lateral view. Fig. 3.5a, b Lingular pneumonia. Note the posterior boundary of the major fissure (a), the indistinct cardiac outline, and the translucent costophrenic angle (b). There may be associated consolidation of the anterior segment of the left upper lobe. Fig. 3.6a, b Bronchopneumonia. Diffuse reticulonodular shadowing is seen in the right lower zone. Radiograph taken after 10 days of antibiotic therapy shows complete resolution of change. Fig. 3.7a, b Postictal aspiration pneumonia (a) with clearing of consolidation on follow-up radiograph taken 8 weeks later following prolonged antibiotic therapy (b). Fig. 3.8a, b Interstitial pneumonia. Radiograph shows increased perihilar linear markings in a patient who presented with cough, fever, hoarseness, and elevated mycoplasma antibody titers. Parapneumonic Pleural Effusion Pleural fluid usually collects adjacent to lobar pneumonia and causes homogeneous opacification of the pleural space. The effusion may form a spindle-shaped mass within the interlobar fissure, or it may be freely mobile and gravitate to the costophrenic sulcus, causing blunting of the costophrenic angle. CT is rarely used in the investigation of community-acquired bacterial pneumonia; the diagnosis is usually based on clinical and plain radiographic findings. However, CT accurately demonstrates the extent of the pneumonia and allows for earlier detection than the plain radiograph. It also demonstrates complications such as pulmonary abscess formation and development of empyema. Ground-glass opacification refers to a “hazy” increase in lung attenuation that does not obscure of pulmonary vessels. It may indicate interstitial thickening that is below the resolution of CT or partial filling of the alveoli. Air space nodules range from 3 to 10 mm in diameter and probably represent peribronchiolar consolidation (Webb 1989, Murata 1986). They tend to be centrilobular in distribution but may spread and coalesce to give more extensive areas of patchy consolidation. A lung abscess is a circumscribed area of inflammation with purulent liquefaction (Fig. 3.9). It may progress rapidly and erode into an adjacent bronchus or into the pleura. In this era of antibiotic therapy, abscess formation has become much less common though it remains a serious complication of pneumonia with reported mortality rates of 20–50%. Staphylococcal or Klebsiella pneumonia may be complicated by abscess formation. Abscesses also develop as a result of aspiration, as a complication of infarction, bronchiectasis, or distal to bronchial obstruction. Multiple abscesses may also be a manifestation of septic lung emboli; this may be seen in the setting of a nidus of infection elsewhere (Fig. 3.10). Right-sided bacterial endocarditis including tricuspid valve infection in intravenous drug users, infected venous catheters, and bacterial pharyngitis/tonsillitis in the setting of Lemierre syndrome are recognized causes of septic pulmonary embolism (Fig. 3.11, 3.12). The yellowish, purulent focus of the abscess is surrounded by a seropurulent exudate in the surrounding alveoli. In favorable cases the pus is expectorated and the process heals by scarring. In other cases residual cavities persist and may become colonized by Aspergillus fumigatus or other organisms. In severe cases the abscess may rupture into the pleural cavity, leading to formation of a pyopneumothorax. In children, the residual lung cavity may hyperinflate via a check-valve mechanism, resulting in a pneumatocele which usually resolves in 4–6 weeks. Fig. 3.9 Lung abscess. Fig. 3.10 Septic pulmonary emboli resulting from systemic sepsis in pyelonephritis. Fig. 3.11a-c Multiple foci of pulmonary consolidation, some of which show cavitation. Patient had an episode of acute tonsillitis with systemic sepsis. Fig. 3.12 Pneumonia with lung abscess formation in intravenous drug user. CT shows predominantly right-sided consolidation with areas of abscess formation. Symptomatology resembles that of acute pneumonia with fever, rigors, cough productive of purulent sputum, and leucocytosis. Patients with diabetes mellitus, alcoholism, and immunocompromised individuals are at increased risk of developing lung abscess. Most abscesses arise within areas of pneumonic consolidation and are marked by the development of a discrete area of low density necrosis and cavitation. The posterobasal segments of the lower lobes are most commonly involved (see Fig. 3.9). Radiologic progression maybequite rapid. Rupture of an abscess into a draining bronchus produces a cavity with an air-fluid level (Fig. 3.13, 3.14). Multiple cavities developing within consolidated lung is known as necrotizing pneumonia. CT allows earlier detection of abscess formation within areas of pulmonary consolidation than does the standard chest radiograph. CT is also superior in defining the relationship of the process to the pleural cavity, and the following features may help in differentiation from empyema: Empyema tends to be lenticular in shape, and the angle of interface with the chest wall is usually obtuse. A lung abscess is usually spherical and produces an acute angle with the chest wall. Empyema fluid lies between thickened parietal and visceral pleura (the split pleura sign; Fig. 3.15). This pleural thickening is relatively smooth in contrast to the wall of an abscess, which may be thickened, quite irregular, and contain locules of gas. Lung adjacent to an empyema is compressed with pulmonary vascular displacement. A lung abscess is associated with destruction of pulmonary parenchyma. Fig. 3.13a-d Lung abscess (a, c) with resolution after 11 weeks’ therapy (b, d). Radiograph a showed consolidation with possible cavitation. CT (c) confirms the presence of an abscess. Fig. 3.14a, b Postpneumonic lung abscess with an air-fluid level. Causative organism: staphylococcus. Fig. 3.15 CT shows large right-sided empyema with “split pleura” sign. Tuberculosis is an infectious disease which may affect any organ but shows a definite predilection for the lungs. In 95% of cases the causative organism is Mycobacterium tuberculosis humanus. A less common strain is Mycobacterium bovis. Atypical mycobacteria such as M. kansasii and M. balnei occur only sporadically. The incidence of another atypical mycobacterial infection, mycobacterium avium complex (MAC), increased markedly in the 1980s and 1990s mainly due to the increasing number of patients with acquired immunodeficiency syndrome (AIDS) and depleted helper T-lymphocyte CD4 counts (see p. 97). In 1900, tuberculosis was still a worldwide epidemic with a mortality rate of approximately 250 per 100 000 per year. Effective antituberculous therapy and better socioeconomic conditions have substantially reduced the incidence and prevalence of tuberculosis, with a resulting decline in mortality rates. Mortality rates in Germany are less than 3 per 100 000 per year. In the United States, 1800 tuberculosis-related deaths are reported each year, corresponding to a mortality rate of less than 1 per 100 000 per year. There was a steady decline in the incidence of tuberculosis in industrialized countries during the second half of the 20th century until the mid 1980s. In Germany, the incidence had fallen from 174 new cases per 100 000 in 1942 to less than 32 new cases per 100 000 population in 1994 (1994 Report of the German Central Committee on Tuberculosis Control). However, in the late 1980s and early 1990s, this downward trend reversed, due mainly to the increasing numbers of cases in patients with AIDS (Im 1995). In the United States, there were approximately 20 000 new cases in 1985; this had increased to more than 25 000 new cases by 1990. More recently, the resurgence of tuberculosis in Western countries has also been attributed to immigration and the development of multidrug-resistant disease (MDR-TB; Faustini et al. 2006). The incidence and prevalence of tuberculosis has always remained high in endemic regions and is the leading cause of death in patients with AIDS in developing countries. The main factor determining whether tuberculous infection progresses to disease is the immune competence of the individual (Murray 1996). Today the disease most commonly is found in persons whose immune status is compromised by old age, alcohol abuse, diabetes mellitus, steroid therapy, or AIDS. There is also a relatively high incidence in certain ethnic groups, many of whom have recently immigrated to Western Europe and North America. Tuberculosis is classically divided into primary and postprimary disease (Fig. 3.16). Some controversy exists as to whether the latter represents reactivation or reinfection (McAdams et al. 1995). Primary tuberculosis occurs in those not previously exposed to M. tuberculosis, is frequently asymptomatic, and therefore is not detected clinically. Postprimary or “cavitating” tuberculosis occurs in previously sensitized individuals; before the advent of antituberculosis chemotherapy, it was frequently fatal (galloping consumption). Today, fibrocirrhotic end-stage disease with severe ventilatory impairment may lead to eventual death from decompensated cor pulmonale. The frontal chest radiograph remains the initial imaging investigation in tuberculosis. Some studies have emphasized the role of high-resolution CT, particularly in the detection of endobronchial spread (Lee 1991, Im et al. 1993, Hatipoglu et al. 1996). Bacteriological diagnosis is made from detection of acid-fast bacilli (AFB) in sputum, gastric washings, pleural fluid and, in patients proceeding to bronchoscopy, from bronchoalveolar lavage (BAL) fluid. Newer immunologic and nucleic acid-based techniques are also emerging (Furin and Johnson 2005). Fig. 3.16 Pulmonary tuberculosis. The German pathologist K. E. Ranke identified three stages in the evolution of tuberculosis. In modern nomenclature, the last two Ranke stages are included in the postprimary phase (Table 3.1 and Fig. 3.16). Table 3.1 Stages of pulmonary tuberculosis (from Doerr, Schmidt, Schmincke) Fig. 3.17 Frequency distribution of tuberculous lesions by lung segments (Doerr 1983). Inhaled tubercle bacilli initially evoke a focal, nonspecific subpleural alveolitis which converts to a tuberculosis-specific inflammatory focus in about 10 days (Ghon focus). The latter is characterized by central colliquative necrosis, also termed caseous necrosis due to its grayish-yellow appearance. There is surrounding granulation tissue rich in lymphocytes, epitheloid cells, and Langerhans giant cells. Spread of tubercle via the lymphatics leads to a specific hilar lymphadenitis. In the great majority of cases, this primary complex (Ghon focus + regional lymphadenitis) heals with fibrosis and may calcify. Large infected lymph nodes may compress the bronchi, particularly the right middle lobe bronchus, with resulting distal atelectasis; this occurs almost exclusively in children (epituberculosis). In the severely immunocompromised patient, caseous lymphadenitis may erode into an airway resulting in tuberculous dissemination through primary endobronchial spread. Mycobacteria entering the blood from the primary complex may become disseminated to numerous extrapulmonary sites (urogenital system, bones, meninges, adrenals, bowel, etc.). This form of postprimary tuberculosis is characterized by cavitating lesions in the upper lobes or in the apical segments of the lower lobes (Fig. 3.17). Rupture of a parenchymal focus into an adjacent airway and subsequent endobronchial spread may lead to extensive pulmonary involvement. Fig. 3.18a, b Acute primary complex. The hazy infiltrate in the right upper lobe, lymphatic stranding, and lymphadenitis form a dumbbell-shaped configuration. Primary tuberculosis usually is asymptomatic. Occasionally, low-grade pyrexia with night sweats, coughing, anorexia, and erythema nodosum develop. With progressive postprimary tuberculosis, the above clinical manifestations are present together with hemoptysis and dyspnea. The tuberculin skin test is positive, and acid-fast bacilli may be detected in the sputum, gastric washings, pleural and bronchoalveolar lavage fluid. Primary tuberculosis is rarely detected on the chest radiograph. Positive radiographic findings are present in only about 20% of children with a positive tuberculin skin test: Fig. 3.19 Calcified primary complex, considered a normal incidental finding. Hematogenous Dissemination Postprimary/secondary pulmonary tuberculosis produces a spectrum of radiographic manifestations; exudative, productive, cavitary, and fibrotic changes frequently occur simultaneously. Because of the predilection for the apical and posterior segments of the upper lobe and the apical segment of the lower lobe, parenchymal changes in these regions in the correct clinical setting should arouse suspicion of tuberculosis. Fig. 3.20a, b Miliary tuberculosis with fine nodular shadowing throughout both lungs. Fig. 3.21 Tuberculous pleural effusion. Mycobacteria were cultured from the lymphocyte-rich pleural aspirate. Fig. 3.22a, b Tuberculosis. Plain radiograph (a) shows bilateral reticulonodular shadowing. CT (b) shows features of endobronchial spread. Fig. 3.23 Multiple tuberculomas. Fig. 3.24 Exudative cavitating tuberculosis with areas of caseous pneumonia and liquefaction. Fig. 3.25 Fibrocirrhotic pulmonary tuberculosis. Upper lobe destruction and fibrosis are associated with elevation of the hila and compensatory emphysema in the lower zones. Fig. 3.26 Tuberculous pleural thickening and fibrocirrhotic pulmonary change. CT manifestations of tuberculosis include: Fig. 3.27a-d CT appearances of tuberculosis. a Features of active postprimary tuberculosis with cavitating lesion in apical segment of left lower lobe and adjacent nodular shadowing in apicoposterior segment of left upper lobe. b Tuberculosis with features of endobronchial spread including centrilobular nodules, branching linear structures (tree-in-bud appearance), and confluent poorly defined nodules. c CT shows bilateral fine nodular shadowing consistent with military TB. d CT shows “healed” cavity with fibrosis in left upper lobe but with evidence of reactivated disease with cavitation and features of endobronchial spread in right upper lobe. Fungal disease of the lung may be classified as endemic or opportunistic. Endemic fungal diseases are caused by pathogenic fungi in an immunocompetent individual. They include histoplasmosis, coccidioidomycosis, blastomycosis, and sporotrichosis. These infections are endemic in the U.S., Africa, and Asia and are seen sporadically in Europe as a result of foreign travel. Opportunistic fungal infection (aspergillosis, candidiasis) is caused by saprophytic fungi, which usually are present in the oral mucosa and become pathogenic in the immunocompromised host. These pneumomycoses have been encountered more frequently since the advent of antibiotics and chemotherapy. However, the overall incidence of pulmonary fungal infections remains low. The clinical symptoms and radiographic findings of these diseases usually resemble those of bacterial pneumonia. Thin section/high resolution CT, in some cases, may be helpful in suggesting the diagnosis. Definitive diagnosis, however, is dependent on identification of the fungus at microscopy. Candida albicans is part of the normal human microbial flora of the oral cavity. Pulmonary candidiasis occurs only in the immunocompromised individual. Fig. 3.28 Candida pneumonia in a leukemic patient on chemotherapy, who presented with oral candidiasis. The right upper lobe continued to show patchy consolidation for several weeks, and two smaller cavitating foci developed on the left side. Pulmonary candidiasis should be suspected in the presence of a pneumonia that is refractory to standard therapy or in the immunocompromised host with florid oral or esophageal candidiasis. The diagnosis may be established by demonstration of candida in transbronchial biopsy specimens. Sputum analysis is of no value because of the ubiquitous nature of the organism (Geary et al. 1980). A wide spectrum of radiographic findings has been described in candida pneumonia. Appearances may be indistinguishable from that of bacterial pneumonia with lobar or segmental consolidation (Fig. 3.28). Diffuse bilateral alveolar or mixed alveolar-interstitial shadowing may be seen (Buff et al. 1982; Fig. 3.29). Candida pneumonia may present as multiple small pulmonary abscesses; these may be randomly distributed if hematogenous spread has occurred or peribronchiolar when resulting from aspiration (Müller1990). A miliary-nodular pattern has been described in pulmonary candidiasis (Pagani 1981), and diffuse pulmonary hemorrhage is also recognized as a manifestation (Müller1991). Aspergillus fumigatus, A. flavus, and A. niger are ubiquitous and flourish in substances such as cereal grains. They also constitute part of the flora of the healthy oral cavity. The following are recognized manifestations of aspergillosis. Fig. 3.29 Pulmonary candidiasis may present as lobar pneumonia, interstitial pneumonia, or bronchopneumonia with cavitation. Primary invasive aspergillosis develops when massive amounts of fungal spores are inhaled, usually from cereal dust. The hosts have normal immunity.
Pneumonia
Pathology
Lobar Pneumonia
Bronchopneumonia (Lobular Pneumonia)
Interstitial Pneumonia
Clinical Features
Radiologic Findings
Plain Chest Radiograph
Computed Tomography (CT)
Lung Abscess and Septic Pulmonary Emboli
Pathology
Clinical Features
Radiologic Findings
Plain Chest Radiograph
Computed Tomography
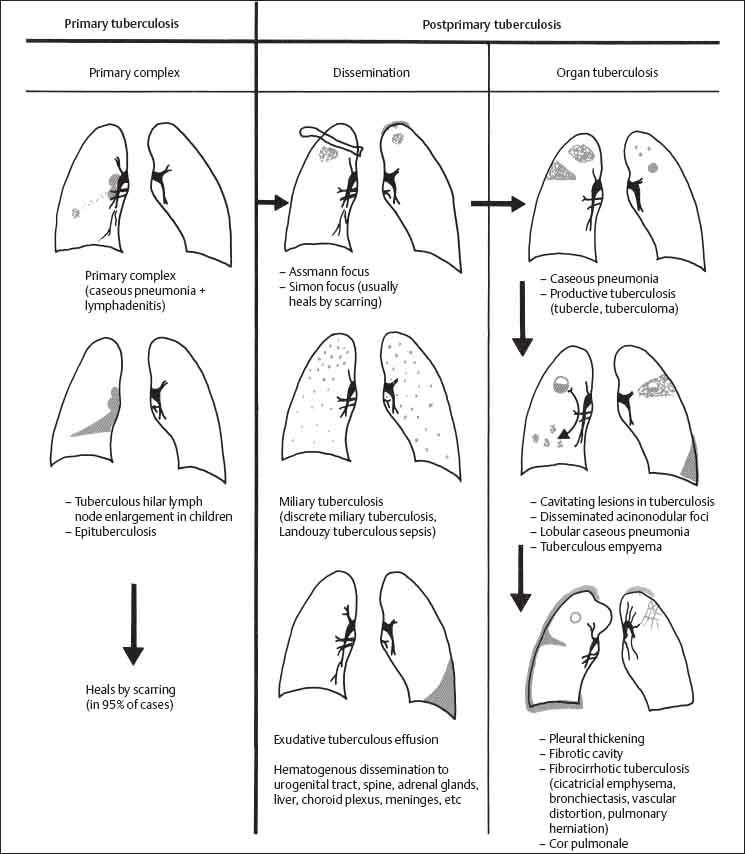
Pulmonary Tuberculosis
Pathology
A. Primary stage
Primary pulmonary focus (Ghon focus) and regional lymphadenitis = primary complex (dumbbell-shaped consolidation as described by K. E. Ranke)
B. Postprimary stage
Primary Complex
Hematogenous Dissemination
Postprimary Organ Tuberculosis
Clinical Features
Radiologic Findings
Chest Radiograph
Computed Tomography
Fungal Diseases of the Lung
Candidiasis
Clinical Features
Radiologic Findings
Aspergillosis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree