Chapter 5 INFECTION OF THE MUSCULOSKELETAL SYSTEM
Infection constitutes one of the true musculoskeletal imaging emergencies. Imaging appearance depends on the anatomic location, infecting organism, and modality used. Infection is common, and radiologists should be familiar with its common and atypical manifestations. Infection can mimic other conditions radiologically, so one should always consider the diagnosis as part of the differential for an unknown condition.
MODES OF SPREAD
Appendicular Skeleton
Because of rich synovial vascularity, the joints can be hematogenously implanted with infection at any age. The pattern of hematogenous osteomyelitis in the appendicular skeleton varies with age (Fig. 5-1). In the infant, metaphyseal nutrient vessels pass through the growth plate to the epiphysis, and osteomyelitis often involves the epiphysis. In children and adolescents, blood vessels are excluded from the more mature growth plate, and organisms deposit preferentially in the metaphyseal capillaries; this explains the metaphyseal location of Brodie abscesses. After the growth plate closes, vessels again extend to the epiphysis. After hematogenous infection, the process can spread contiguously along fascial planes to adjacent structures. The infectious material can also dissect through to the skin surface, creating a sinus tract and leading to spontaneous drainage.
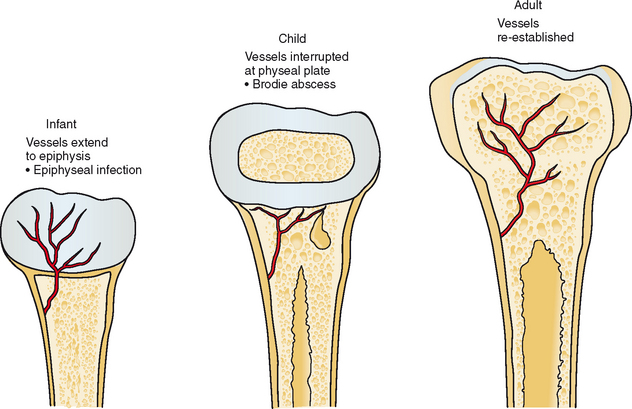
Figure 5-1 Vascular distribution during development and influence on sites of hematogenous infection.
In the diabetic foot, contiguous spread is far more common than hematogenous spread. Diabetic patients acquire ulcers through which infection (typically multiorganism) spreads to underlying bones and joints. This mode of spread is also very common in paralyzed patients, occurring at areas of pressure or friction such as the ischial tuberosities, sacrum, greater trochanters, and posterior calcaneus.
DISEASE CATEGORIES AND FINDINGS
Cellulitis
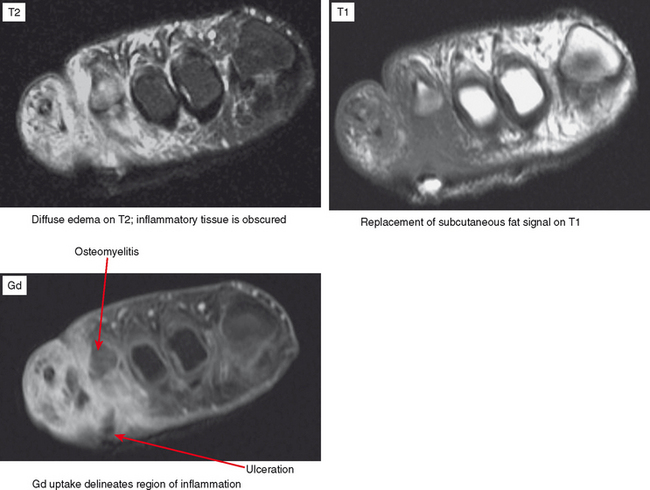
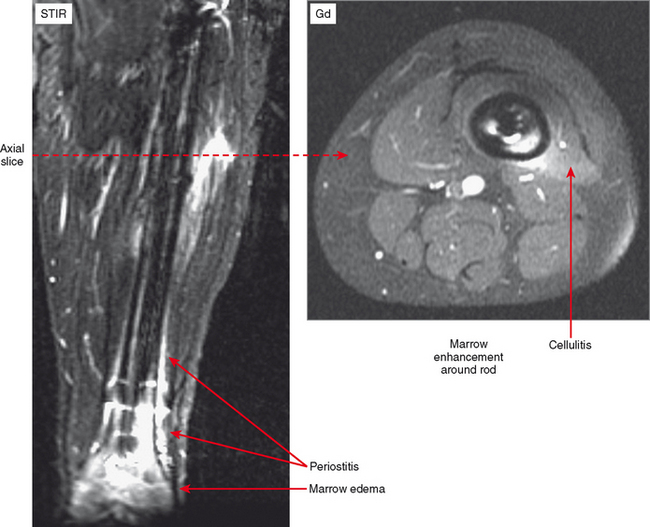
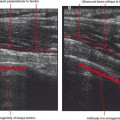
Cellulitis is an acute infection of the subcutaneous tissues; the diagnosis is most often clinical. However, MR imaging is helpful for evaluating the underlying tissues for such findings as myositis, abscess, sinus tract, fasciitis, and osteomyelitis. MRI is also useful for determining the extent of involvement of the infective process. Contrast enhancement is extremely useful for differentiating cellulitis from noninflammatory edema and also for delineating abscesses and sinus tracts that characteristically rim-enhance. Cellulitis results in diffuse regional enhancement, whereas bland or dependent edema demonstrates no more enhancement than the surrounding tissues, even when extensive (Fig. 5-2).
On MRI, infection—whether in soft tissue or bone—results in metabolization of fat. Hence, low T1 signal is observed on MRI in conjunction with the high T2 signal and enhancement that characterize the inflammatory process. Since it takes some time for this resorption to occur, in the very early stages of infection (cellulitis and osteomyelitis) fat in the soft tissues or marrow may not be completely replaced, but there may be only slightly lower T1 signal than the surrounding tissues. Therefore, although replacement of fat signal is more specific for infection in the appropriate clinical setting, edema on T2 or short T1 inversion recovery (STIR) or enhancement is more sensitive.
Pyomyositis and Infectious Fasciitis
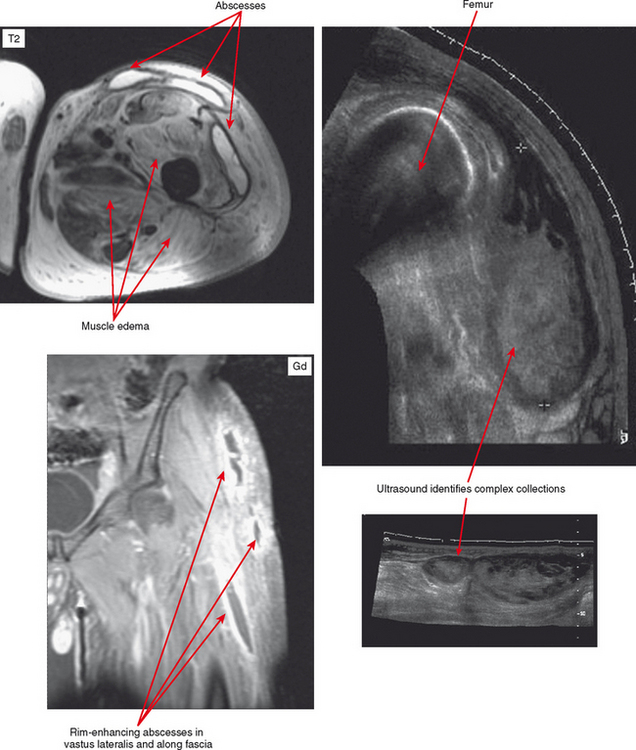
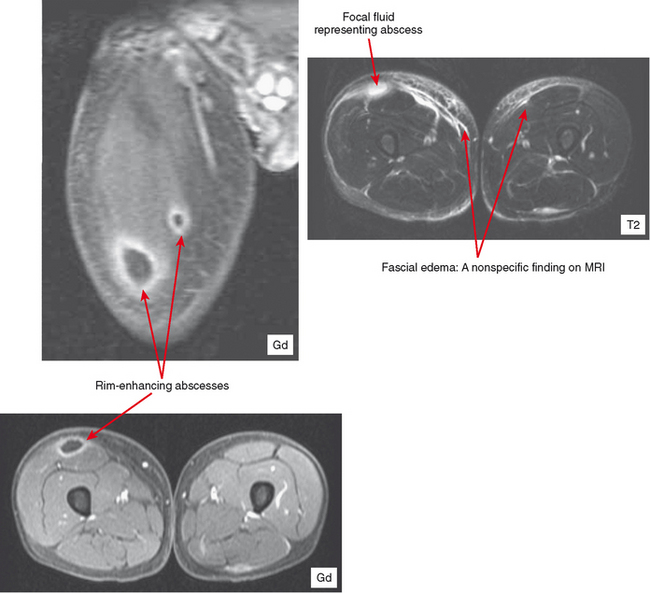
MRI is highly sensitive, with hyperintensity on T2-weighted or STIR images being initially diffuse. On T1-weighted images, the muscle may appear enlarged, but signal may appear relatively normal. Perifascial infiltration may be seen on T1- and T2-weighted sequences. Post-contrast images show diffuse enhancement in the early stages and help to separate this process from other entities such as diabetic myonecrosis. However, in later stages superimposed necrosis may also occur, and abscess formation can result in heterogeneous enhancement. With disease progression, more focal fluid collections are evident. Post-contrast images demonstrate rim enhancement of these small abscesses. Areas of abscess and necrosis often require debridement. Contrast-enhanced sequences can provide the surgeon with a roadmap for treatment. Ultrasound can also be useful to detect abscess formation (Fig. 5-3).
Muscle edema on MRI is nonspecific, with many differential diagnostic possibilities including injury, denervation, rhabdomyolysis, infarction and diabetic myonecrosis, autoimmune myositis, and tumor. Therefore, clinical correlation is essential (see Chapter 15).
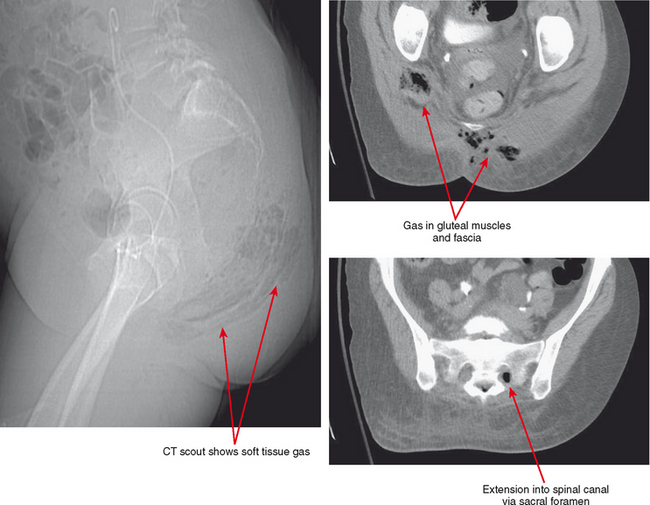
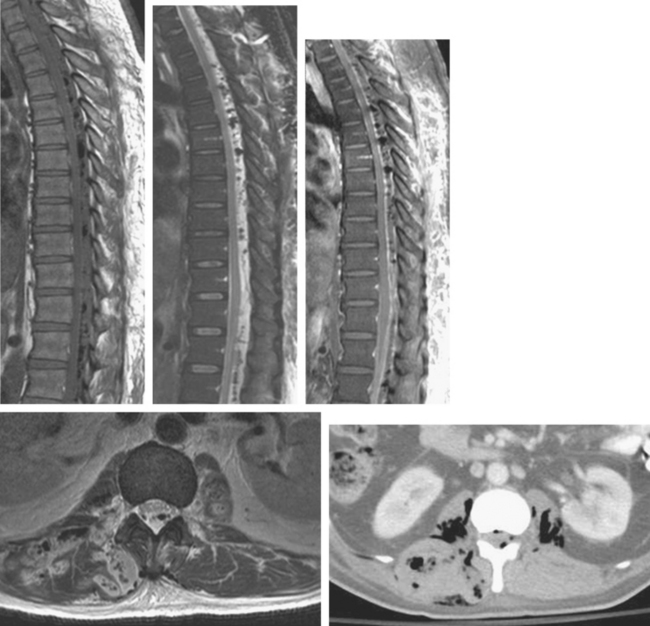
Infectious fasciitis is a true surgical emergency, requiring immediate debridement and decompression as well as aggressive antibiotic therapy. Although MRI is very sensitive for evaluation of fascial inflammation, fascial edema and even enhancement are not specific for clinically aggressive fascial infection. In addition, fascial gas seen in aggressive cases may be difficult to see on routine MRI sequences, and delay in acquisition of the scan may preclude MRI as a sufficient diagnostic tool in this setting. CT or even radiographs may be more useful in this regard, owing to wide availability, rapid acquisition, and high sensitivity for fascial gas (Figs. 5-4 and 5-5). MRI remains useful for evaluating the extent of infection and the presence of underlying abscess, septic arthritis, and osteomyelitis.
Abscess
An abscess is a localized collection of necrotic tissue, inflammatory cells, and neutrophils, walled off by a highly vascular and typically irregular inflammatory pseudocapsule. A variable degree of surrounding soft tissue edema/cellulitis surrounds the process. Before liquefaction, an abscess is referred to as a phlegmon, an ill-defined inflammatory region of the subcutaneous or deep soft tissues without discrete fluid collection. With maturation, the infected tissue undergoes necrosis and liquefaction, thus becoming an abscess, with a peripheral hypercellular and hypervascular zone, particularly in the acute phase.
On MRI, the central cavity of an abscess is hypointense on T1-weighted images, but apart from asymmetric prominence of the soft tissues the abscess may not be apparent since signal can be similar to that of adjacent muscle. Most abscess collections are hyperintense on T2-weighted or STIR images with a variable degree of surrounding soft tissue edema. This edema may be intense, and, again, the abscess cavity may blend with surrounding tissues. Fluid-sensitive sequences tend to show heterogeneous signal within the abscess, reflecting the necrotic tissue within. The margin, composed of hypervascular inflammatory tissue, demonstrates thick enhancement after contrast administration. The central portion remains hypointense, making the abscess cavity stand out on fat-suppressed post-contrast images (Fig. 5-6). The multiplanar capability of MR and soft tissue contrast make it the ideal modality in planning before surgery or percutaneous drainage.
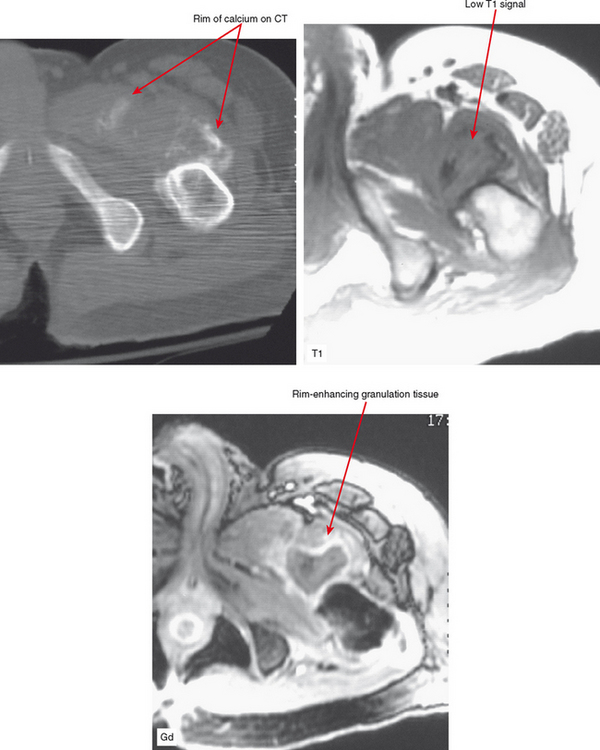
Heterotopic bone formation, as seen in spinal cord injury and following trauma or surgery, may be confused in its early stage of development with abscess formation because these foci are also often T2-hyperintense and rim-enhanced after gadolinium administration. Careful interpretation is warranted in the spinal cord injury patients, who are concomitantly at increased risk of decubitus ulcers and thus abscess formation. Gradient-echo images, demonstrating blooming artifact, may be of assistance in differentiating between these two pathologic processes by MRI. CT is helpful for detecting subtle marginal calcifications seen in early heterotopic ossification (Fig. 5-7).
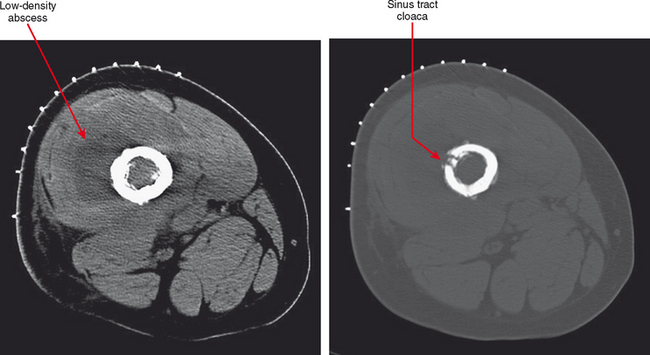
CT or ultrasound can be obtained alternatively for suspicion of abscess, especially if MRI is contraindicated. CT should be performed for this purpose with contrast to delineate the rim enhancement; this appearance is similar to that on MR images, but much more faint. On CT, fluid is different in density from muscle, but complex fluid seen in abscesses, especially in the presence of hemorrhage, can make small collections of fluid difficult to see. Rim enhancement helps to detect such collections and delineate extent. Sinus tracts and abscesses that communicate with the skin may also fill with air, and portions may be visible based on this finding. Ultrasound is excellent for detection of fluid collections, and power Doppler can identify the hyperemia of the pseudocapsule and surrounding tissues that results in rim enhancement on other modalities. Ultrasound is the modality of choice for aspirating such collections. However, ultrasound is limited compared with contrast-enhanced MR with regard to the overall picture as to extent of the inflammatory process affecting various tissues (see Fig. 5-3).
Septic Arthritis and Septic Bursitis
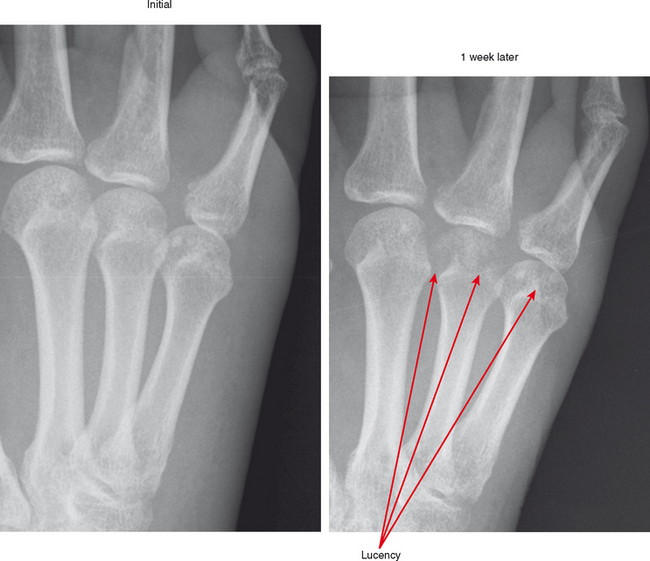
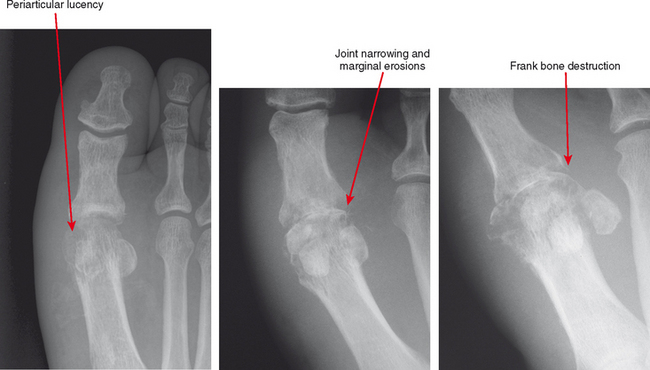
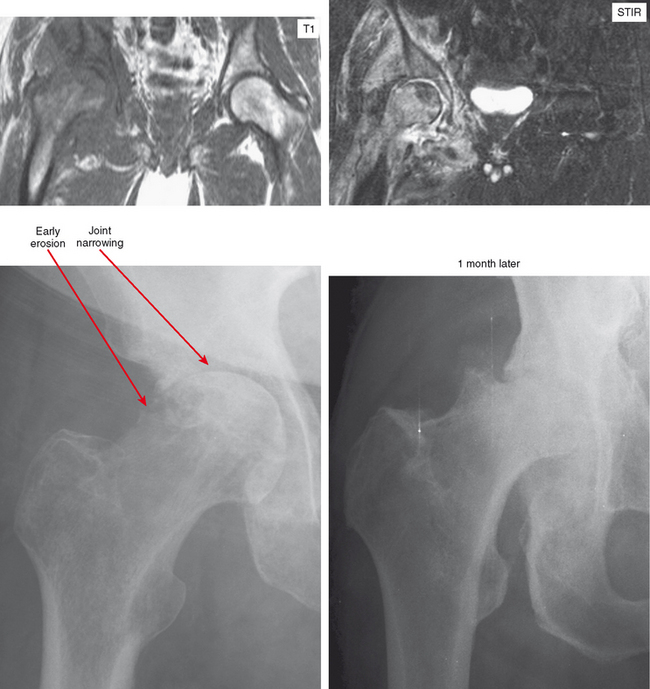
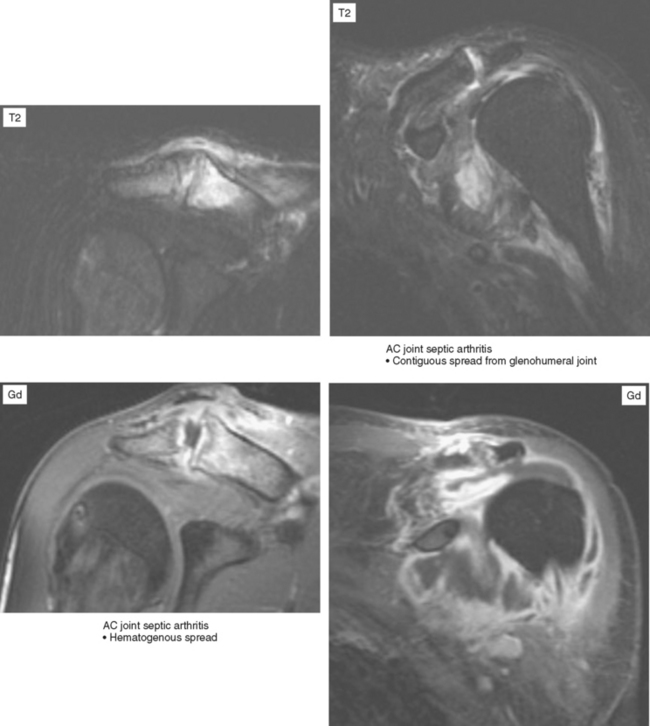
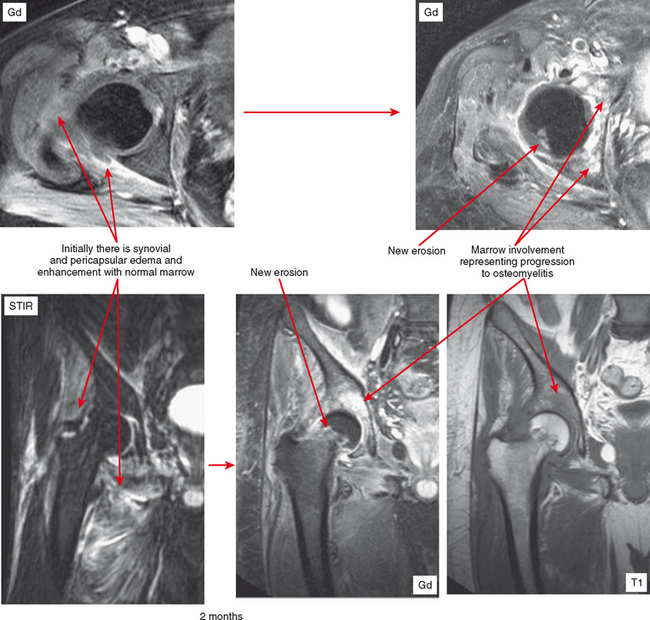
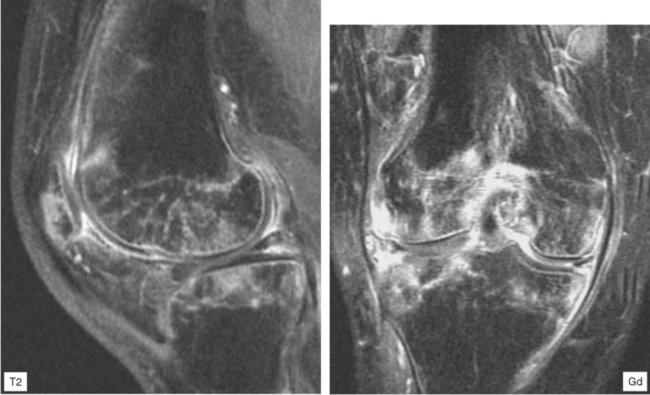
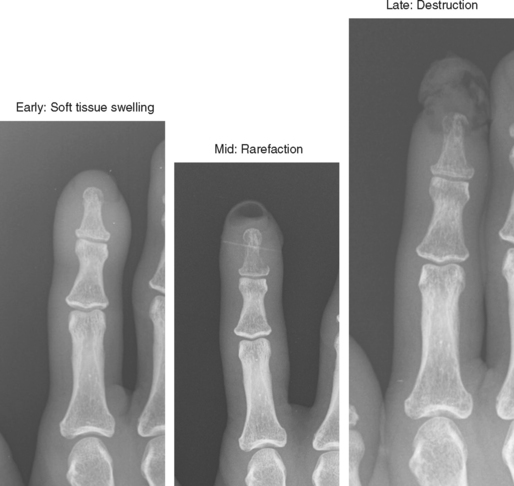
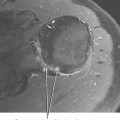
Joint effusion is a hallmark of septic arthritis, whereas focal soft tissue swelling in the region of an anatomic bursa or over a bony prominence may indicate septic bursitis. In both, fluid is often complex on MRI. Complex fluid is also a characteristic of other noninfectious inflammatory arthropathies such as rheumatoid arthritis, gout, psoriasis, and, to a lesser degree, even osteoarthritis; unless clinical information is available, it may be impossible to tell the difference (see Chapter 4, Arthritis). Conversely, whenever a monarticular arthropathy is encountered, infection should be excluded. On radiographs, the only early sign is an effusion and juxta-articular soft tissue swelling, which, depending on the joint, may be difficult or impossible to detect (Figs. 5-8 through 5-10). Like abscesses, septic arthritis and bursitis on MRI demonstrate thick rim enhancement. Because of hyperemia, rarefaction (decreased density) of the subchondral bone next occurs, seen as subchondral edema on MRI (Figs. 5-11 through 5-13). Erosions begin at the margins of the joint, again as in rheumatoid arthritis, but while the infection progresses frank destruction of the articular surfaces may occur.
In some joints, erosions and effusion are difficult to see on radiographs, such as the sacroiliac joint. For the sacroiliac joint look at the inferior aspect of the joint (the synovial portion) and see whether there is loss of the thin white line, which represents the articular surface seen tangentially. This is the characteristic appearance of erosion in this location. Cartilage destruction (inflammatory breakdown products are chondrolytic) eventually results in joint narrowing, radiographically visible in later stages. As above, linear subchondral edema is common on MRI in septic arthritis. However, when the edema or enhancement extends beyond the subchondral bone into the medullary cavity, in the appropriate clinical setting osteomyelitis should be considered.
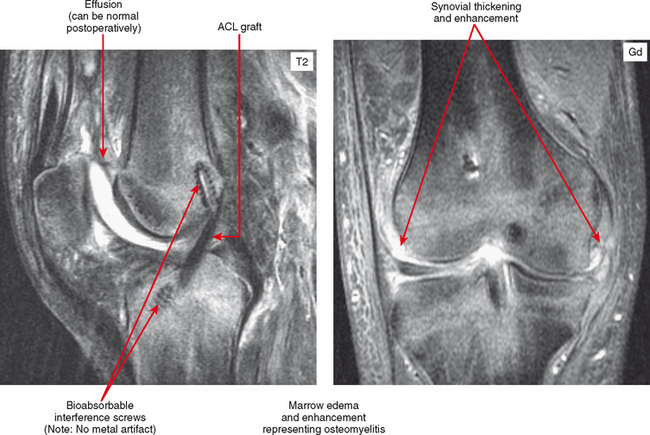
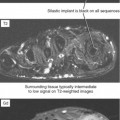
After surgery, joints may also have joint effusion and synovial proliferation, and shortly after surgery—even arthroscopic surgery—the hyperemic synovium can simulate infection. The soft tissues are often very swollen and periarticular fluid collections can also be seen, depending on the surgical approach and extent. Labeled white blood cell (WBC) nuclear medicine scan can show uptake, but if there is clinical suspicion of infection at this point, aspiration may be the best overall diagnostic option. Nevertheless, MRI can be useful to exclude medullary marrow edema or enhancement associated with osteomyelitis (Figs. 5-14 and 5-15). Unless there has been extensive medullary drilling or exposure, typically the marrow signal abnormality is limited to the immediate surgical zone and signal abnormality in remote areas should be considered suspicious. For example, shortly after anterior cruciate ligament reconstruction there will be edema limited to immediately around the tunnels and in the subchondral bone. Reactive synovitis after surgery can clinically simulate infection and often prompts MRI requests. Some implants, especially bioabsorbable components (e.g., screws, anchors, sutures) can incite an inflammatory response. MRI shows a very complex effusion and subchondral marrow edema. One clue that a bioabsorbable screw was used is a low signal focus without any metallic artifact. The bioabsorbable components are invisible on radiographs, and without provided history, surgical changes are subtle and may be overlooked. Again, it may be necessary to aspirate the joint in this setting if infection is suspected.
Osteomyelitis
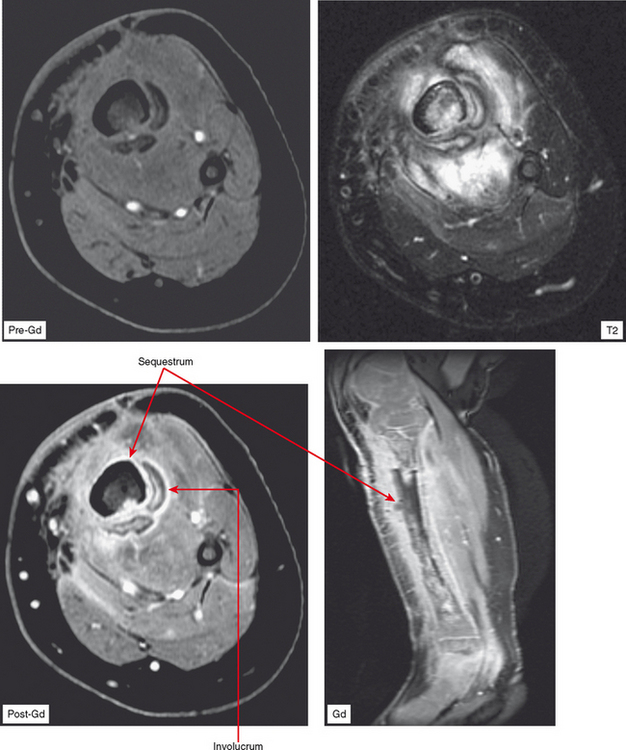
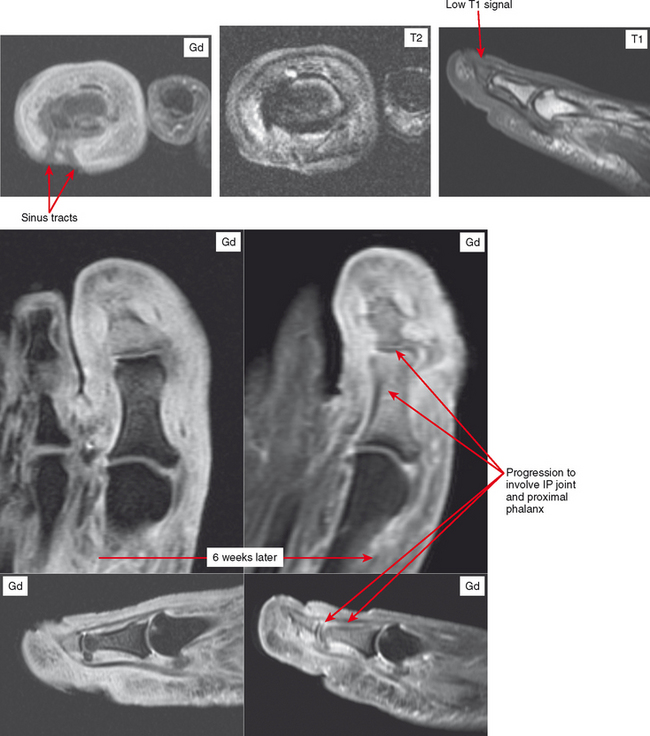
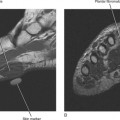
Osteomyelitis refers to infection of the medullary portion of the bone marrow, whereas osteitis indicates involvement of the surface of the bone only. A sequestrum represents a segment of necrotic bone that is separated from living bone by granulation tissue. An involucrum denotes a layer of living bone that has formed about the dead bone. An opening in the involucrum is termed a cloaca (Figs. 5-16 and 5-17). A Brodie abscess is an intraosseous abscess in children usually caused by Staphylococcus aureus infection and represents a subacute or chronic infection (Fig. 5-18). Chronic osteomyelitis occurs under areas of chronic ulceration or after trauma or surgery with unsuccessful or inadequate treatment of acute bone infection. The infection may smolder for many years, occasionally presenting with drainage through sinus tracts and acute-on-chronic osteomyelitis. Necrotic bone (e.g., sequestrum) is often colonized with organisms even after effective treatment, and must be debrided to avoid re-emergence of active infection. Chronic recurrent multifocal osteomyelitis is a rare disorder primarily involving children and adolescents and characterized by a prolonged, fluctuating course most often involving tubular bones, the clavicle, and less often the spine. No organisms are cultured and it may be a noninfectious condition possibly related to a type of immunocompromised state as yet undefined or an organism such as a virus as yet unidentified.
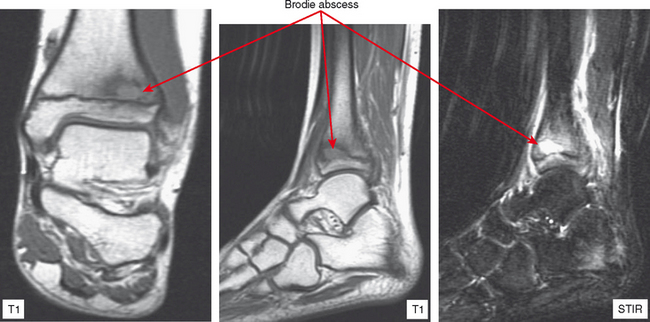
Figure 5-18 A Brodie abscess in an adolescent. Note how the lesion abuts or “drips” toward the physeal plate.
Acute osteomyelitis is difficult to detect on radiographs in the early stages (Fig. 5-19). Adjacent soft tissue swelling is present but is nonspecific and may not be seen at all in deep structures. Cortical rarefaction or resorption is next seen as a region of decreased cortical density or a permeative pattern in the cortex. By the time frank cortical destruction is seen, the infectious process is well established and generally extensive. The classic radiologic appearance is a lytic, permeative process, characteristic of “small round cell” lesions. Radiographs are still useful as the first test to exclude other pathology, to obtain a baseline exam, and to evaluate for calcifications.
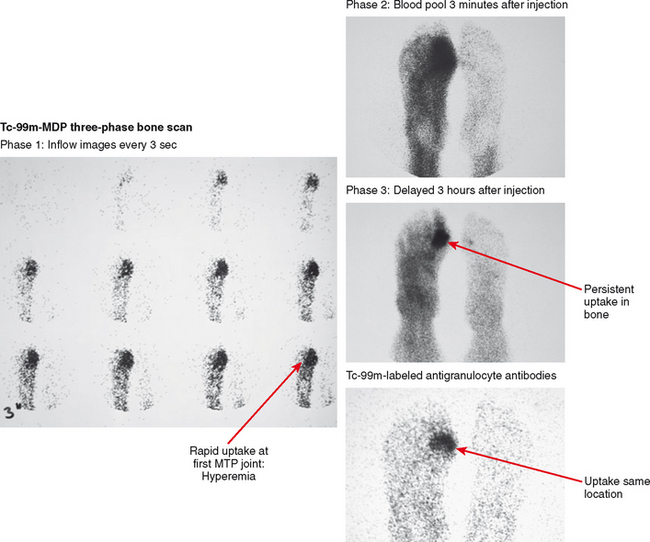
Tc-99m-MDP (methylene diphosphate) three-phase bone scan has high sensitivity for detection of osteomyelitis if there is adequate blood flow to distribute radiotracer. Classically, osteomyelitis presents as rapid uptake on the early inflow phase due to hyperemia, with persistent activity on the second, blood pool phase, and concentration of activity within bone on the delayed phase acquired hours later, indicating increased bone turnover (Fig. 5-20). Uptake on all phases suggests osteomyelitis in the appropriate clinical setting, but these findings can be seen in other inflammatory, traumatic, and neoplastic conditions, as well as neuropathic osteoarthropathy. Increased specificity has been reported with acquisition of a fourth phase, obtained after 24 hours.
Gallium scan, though commonly used for detection of inflammatory processes around the body, has shown limitations in the musculoskeletal system, particularly in the spine and diabetic foot. Instead, when there is a question of infection versus some other process, imaging using WBCs tagged with indium-111 or technetium-99m is recommended. Labeled WBCs are taken up preferentially by inflammatory tissue; this is nonspecific with regard to involved tissue, and cellulitis or abscess results in abnormal activity similar to osteomyelitis. Therefore, the exam is typically interpreted in conjunction with three-phase bone scan, with corresponding uptake concentrating in bone indicative of osteomyelitis. Although the combination is specific, nuclear medicine exams involve a delay and anatomic resolution is low, making these techniques less useful for urgent evaluation (e.g., spine infection) or as preoperative anatomic roadmaps.
On MRI, the classic sign of osteomyelitis is replacement of marrow fat on T1-weighted images, with marrow edema and enhancement on fluid-sensitive and post-contrast images (Fig. 5-21). However, it should be obvious that these findings are not specific for infection and can be seen in a variety of diseases and injuries.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree