Bacteria
Fungi
Parasites
Mycobacteria (Mycobacterium tuberculosis, M. avium)
Spirochetes (Borrelia burgdorferi, Leptospira interrogans, Treponema pallidum)
Brucella
Tropheryma whippelii
Listeria monocytogenes
Neisseria meningitidis
Francisella tularensis
Actinomycetes
Nocardia
Cryptococcus neoformans
Coccidoides immitis
Histoplasma capsulatum
Candida species
Taenia solium (neurocysticercosis)
Amoeba species
Angiostrongylus (eosinophilic meningitis)
Toxoplasma gondii
Clinical manifestations and treatment Septic meningitis is a neurologic emergency with 25% mortality and 60% morbidity. It is characterized by a clinical triad of fever, headache, and stiff neck. Patients look very sick and may become comatose within a few hours. Fever, headache, photophobia, and stiff neck also mark the clinical presentation of aseptic meningitis. Symptoms and complaints are often less severe than in the pyogenic forms, however. Treatment consists of specific antibiotic therapy and supportive care. A 4-day course of dexamethasone may also be prescribed. Subdural empyema is surgically decompressed.
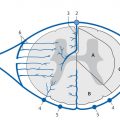
Pathology Infection of the meninges may arise by contiguous spread from an adjacent focus (e.g., sinusitis, otitis), by hematogenous spread from the choroid plexus or cerebrospinal fluid (CSF), and rarely by direct implantation from a penetrating head injury or neurosurgical procedure. The cavernous sinus may also be involved ( ▶ Fig. 5.1). Histologic examination reveals hyperemia of the leptomeninges. In pyogenic meningitis, the dilated capillaries of the pia-arachnoid release massive numbers of neutrophilic granulocytes, which initially fill some of the meshlike spaces of the leptomeninges and later occupy all of the subpial and subarachnoid space. The pus imparts a characteristic yellowish tinge to the brain surface. The ratio of granulocytes to fibrin in the inflammatory exudate depends on the type of causative agent. The inflammation may also involve the blood vessels within the leptomeninges, leading to vasculitis with risk of decreased cerebral blood flow.

Fig. 5.1 Infectious involvement of the left cavernous sinus by inflammation of the sphenoid sinus. CT examination of an adult male with a 5-day history of abducens nerve palsy shows soft-tissue obstruction of the left sphenoid sinus with wall thickening and sclerosis consistent with chronic paranasal sinusitis, also disruption of the lateral wall (a). Enhancement of the cavernous sinus on MRI indicates contiguous spread of inflammation from the sphenoid sinus (b,c).
(a) Axial CT with a bone window. (b) Axial T1w fat-suppressed MR image after contrast administration. (c) Coronal T1w fat-suppressed MR image after contrast administration.
MRI findings
Note
The MRI findings in aseptic meningitis are usually normal.
The leptomeninges or pachymeninges do not necessarily enhance, especially in the early stage. Contrast enhancement is generally absent in viral forms of meningitis. Thickening of the meninges, which is best demonstrated on contrast-enhanced T1w images and often on T2w and PDw images as well, is more common in septic and chronic meningitis ( ▶ Fig. 5.2) than in viral forms. If edematous signal changes are also present in adjacent brain tissue with hyperintensity on FLAIR, T2w, and PDw images, this means that the inflammation has spread to the brain parenchyma, causing encephalitis. High-grade stenoses in larger blood vessels are usually demonstrable by MR angiography (MRA). These vasculitic changes are especially common in predominantly basal tuberculous meningitis and chiefly affect the territory of the middle cerebral artery (MCA), especially the main trunks. Doppler ultrasound is the current modality of choice for the screening and follow-up of intracranial vascular stenoses. MRI is excellent for the early detection of brain infarction due to meningitis-induced vasculitis. Even small acute infarcts appear hyperintense on diffusion-weighted imaging (DWI) and hypointense on apparent diffusion coefficient (ADC) maps (cytotoxic brain edema). Imaging is generally performed before any necessary CSF sampling in order to assess the risk of lumbar puncture. If neuroimaging shows no signs of increased intracranial pressure such as obliterated basal cisterns or effaced extra-axial CSF spaces, lumbar puncture can generally be performed without risk of inducing a herniation. The most important complication of pyogenic meningitis is subdural empyema. In this condition as well, the adjacent meninges may not enhance in every case, especially in the early stage. The contents of the empyema may be hyperintense, isointense, or occasionally hypointense on FLAIR and T2w images, depending on their protein content ( ▶ Fig. 5.3). DWI, while useful for diagnosing brain abscess, is of limited value with empyema, which is usually difficult to evaluate near the calvarium due to artifacts. It typically produces a signal similar to brain abscess with a decrease in the ADC. Portions of the empyema with a higher protein content may gravitate to dependent areas, forming a visible layer.

Fig. 5.2 Pneumococcal meningitis in a 12-year-old boy. MRI shows bilateral frontopolar subdural fluid collections that are slightly hyperintense to CSF on T2w images (a) and markedly hyperintense to CSF on FLAIR images (b) reflecting an elevated protein content. Intense enhancement of the thickened pachymeninges and leptomeninges is noted in the frontoparietal region (c–e).
(a) Axial T2w image. (b) Axial FLAIR image. (c) Axial T1w image after contrast administration. (d) Coronal T1w image after contrast administration. (e) Sagittal T1w image after contrast administration.

Fig. 5.3 Subdural empyemas secondary to frontal sinusitis in a 40-year-old woman. Material of soft-tissue intensity is present in both frontal sinuses (a), consistent with sinusitis. Subdural masses of fluid signal intensity are noted in the anterior interhemispheric fissure (a) and over the surface of the left cerebral hemisphere (b,c). Components with a higher protein content are best demonstrated on the FLAIR image (b); they are hyperintense to CSF and show occipital layering. The preoperative images shows significant mass effect with a midline shift to the right. The postoperative images (d–f) no longer show a mass, but the FLAIR image (f) shows subacute infarcts demarcating in the territory of the left posterior cerebral artery and in the head of the left caudate nucleus. They are accompanied by a number of other acute brain infarcts, which appear as hyperintensities on DWI (e). The ischemic brain infarcts were attributed to vasculitis. Postoperative leptomeningeal enhancement reflects an accompanying meningitis (d) (with kind permission of Prof. Michael Knauth, Department of Neuroradiology, Göttingen University Hospital).
(a) Axial T2w image before surgical drainage of the empyemas. (b) FLAIR image before surgical drainage of the empyemas. (c) Axial T1w image before surgical drainage. (d) Axial T1w image after surgical drainage. (e) Axial DWI after surgical drainage. (f) FLAIR image after surgical drainage of the empyemas.
Differential diagnosis The causative organism is identified by culture of the CSF. It should be noted that enhancement of the spinal and cranial meninges may also occur in the setting of a low CSF pressure syndrome and after (repeated) sampling of CSF from the (lumbar) CSF space. Other important diseases of the meninges such as carcinomatous meningitis or meningeal involvement by lymphoma are distinguished from an infectious meningeal disease on the basis of CSF findings, history, and preexisting underlying disease. Neoplastic involvement of the meninges tends to show a more nodular than linear pattern of enhancement with irregular thickening of the meninges. Differentiation is required from other infectious diseases such as tuberculous meningitis (see ▶ Tuberculous leptomeningitis) or fungal diseases (see ▶ 5.2.4), and from noninfectious forms of meningitis such as rheumatoid diseases, sarcoidosis, and idiopathic pachymeningitis. Subdural hygromas, unlike subdural empyemas and hematomas, are usually isointense to CSF and, unlike typical subdural empyemas, do not cause enhancement of adjacent meninges.
Pitfall
Thickening of the meninges is not always due to infection or malignant infiltration. Chronic low CSF pressure syndrome is an important differential diagnosis. The correct diagnosis is suggested by the clinical features, which include orthostatic headache.
5.2 Infectious Diseases of the Brain Parenchyma
5.2.1 Viral Encephalitis
More than 100 viruses have been identified as causative agents of encephalitis or meningoencephalitis. The spectrum depends on public immunization and on possible contact with other infected people or animal vectors. ▶ Table 5.2 reviews the principal causative organisms and their patterns of involvement on MRI. The most frequent causative agents of viral encephalitis are herpes simplex virus (HSV), human herpesviruses (HHV) types 6 and 7, cytomegalovirus (CMV), EBV, and varicella zoster virus (VZV). The mumps and polio viruses are of minor causal importance in developed countries.
Causative agent | Disease | Route of infection | Pattern of involvement on MRI |
DNA viruses | |||
HSV type 1 | Herpes encephalitis or meningoencephalitis | Droplet infection or direct contact with saliva, urine, feces Primary infection: gingivostomatitis, neuronal transmission, activation of a latent infection of the trigeminal ganglion | Involvement of infarct and mesial portions of temporal lobe, insular cortex, thalamus, cingulate gyrus, and frontobasal cortex (limbic distribution) Frequent hemorrhagic transformation Frequent contrast enhancement |
HSV type 2 | Neonatal herpes encephalitis | Droplet infection or direct contact with saliva, urine, feces Primary infection: genital herpes infection of neonate, in utero or perinatal | Early phase: diffuse brain edema and hemorrhagic transformation Later: calcifications, decreased brain volume |
HHV type 6 | Predominantly cortical encephalitis | Direct physical contact Primary infection: roseola infantum Route of transmission to CNS is unclear | Cortex |
CMV | CMV encephalitis in immunosuppressed adults | Hematogenous, e.g. by blood products, organ transplantation, droplet infection or direct contact | Subependymal (periventricular) hyperintensities on T2w images with slight mass effect Decreased brain volume |
Congenital CMV encephalitis | Transplacental | Decreased brain volume Parenchymal calcifications, mainly periventricular | |
EBV | EBV encephalitis | Hematogenous route Primary infection: infectious mononucleosis | T2w hyperintensities in the basal ganglia, rarely EBV cerebellitis |
VZV | Varicella zoster viral encephalitis | Droplet infection or direct contact Primary infection: reactivation of chickenpox: herpes zoster (shingles), encephalitis Hematogenous spread of virus to CNS | Vasculitic and demyelinating changes in the brain parenchyma, (hemorrhagic) brain infarcts |
JC virus (papovavirus) | PML | Immunosuppression due to HIV infection, drug effects (e.g., natalizumab), underlying malignant disease, etc. | Patchy or round to oval T2w hyperintensities, confluent over time, in subcortical white matter with involvement of lower cortical layers Predilection for parieto-occipital brain Involvement of subcortical U-fibers |
Smallpox virus vaccines | HIV encephalitis (see below) | HIV infection | Unilateral or bilateral asymmetrical, patchy, multifocal hyperintensities on T2w images Decreased brain volume |
RNA viruses | |||
Rubella virus | Meningitis, encephalitis | Droplet infection or direct contact Primary infection: rubella Hematogenous spread to CNS | Microcephaly, parenchymal calcifications, predilection for basal ganglia |
Arboviruses | TBE | Tick bite Hematogenous spread of virus to CNS | MRI usually normal, may rarely show T2w hyperintensities in thalamus, cerebellum, brainstem, and caudate nucleus |
Japanese B encephalitis | Mosquitoes Hematogenous spread of virus to CNS | T2w hyperintensities in brainstem nuclei, basal ganglia, and thalamus | |
Rhabdoviruses | Rabies encephalitis | Bite of dogs and wild animals Hematogenous spread of virus to CNS | Initial stage: predilection for gray matter Later: diffuse T2w hyperintensities in basal ganglia, thalamic pulvinar, periventricular white matter, hippocampus, and brainstem Advanced cases: diffuse brain edema, hemorrhagic transformation |
Measles virus | Acute encephalitis | Late complication: from months to as much as 10 years after measles infection (slow virus disease) | T2w hyperintensities in subcortical white matter, brainstem, cerebellum |
SSPE | Droplet infection or direct contact Primary infection: measles Hematogenous spread of virus to CNS | Initial findings usually normal, followed by initially diffuse brain swelling Later: T2w hyperintensities in periventricular white matter, brainstem, and cerebellum, involvement of putamen and caudate nucleus End stage: decreased brain volume | |
HIV | Acute aseptic meningitis (early manifestation), chronic encephalopathy | Sexual transmission, blood products | Gradual, progressive decrease in brain volume, usually with symmetrical and patchy confluent or focal white-matter lesions Cortex and U-fibers usually spared |
Arenaviruses | Lymphocytic choriomeningitis and –encephalitis | Rodents | |
Mumps virus (paramyxoviruses) | Meningitis, encephalitis, myelitis | Hematogenous from primary infection (mumps) | |
Poliovirus (enteroviruses) | Meningitis, myelitis | Fecal–oral route | |
Influenza viruses | |||
CMV = cytomegalovirus; CNS = central nervous system; EBV = Epstein–Barr virus; HHV, human herpesvirus; HIV = human immunodeficiency virus; HSV = herpes simplex virus; PML = progressive multifocal leukoencephalopathy; SSPE = subacute sclerosing panencephalitis; TBE = tick-borne encephalitis; VZV = varicella zoster virus. | |||
In principle, the causative agents of encephalitis can gain access to the brain by the hematogenous or neural route. Certain viruses have an affinity for specific cells of the CNS based on their surface characteristics. Meningeal cells, oligodendrocytes, astrocytes, and nerve cells are each affected by different types of virus. For example, herpesviruses proliferate mainly in neuronal and glial cells of the limbic system, while the JC virus, the causative agent of progressive multifocal leukoencephalopathy (PML), predominantly affects oligodendrocytes. Coxsackievirus, echovirus, and mumps virus commonly affect meningeal and ependymal cells but rarely attack nerve cells.
Brain damage in viral encephalitis results both from intracellular viral growth and from the inflammatory immune response of the host. This immune response may be a humoral response directed against the virus itself or a cellular response directed against the cell infected by the virus. The different forms of infectious encephalitis have similar pathologies: The brain tissue appears grossly normal in the early stage. Later the meninges show decreased transparency accompanied by vascular congestion and local or diffuse brain swelling. Microscopically, infiltration by inflammatory cells is typical of all viral encephalitides. Except for HSV type 1 encephalitis, which shows an almost pathognomonic pattern of limbic system involvement on MRI, the radiologic findings in adult viral encephalitis are nonspecific; they are associated with focal or diffuse brain edema in the acute phase and focal atrophy with gliosis in the chronic stage. As a result, neuroimaging usually contributes little to viral identification. Nevertheless, some viral encephalitides have distinctive neuroimaging features that may be helpful in narrowing the differential diagnosis.
5.2.1.1 Herpes Simplex Viral Encephalitis
Epidemiology
HSV type 1 encephalitis CNS inflammation is not uncommon in patients with an HSV type 1 viral infection, and this virus is one of the most frequent causes of meningoencephalitis in immunocompetent adults. The incidence is 1:250,000 population per year, with one-half of patients being under 50 years of age. HSV type 1 encephalitis accounts for 95% of all herpes encephalitis cases. HSV type 2 is responsible for only 6 to 15% of herpes encephalitis cases in adults. Following a primary infection, the virus spreads by a retrograde transneuronal route to the olfactory bulb or along a trigeminal nerve branch to the trigeminal (gasserian) ganglion, where it lies dormant in approximately 70% of patients. In approximately one-third of patients with HSV type 1 encephalitis, the encephalitis already develops during the course of the primary infection. Reactivation of the latent infection, often due to immunosuppression, enables the virus to spread further via dural nerve branches to the anterior and middle cranial fossae. Viral reactivation is occasionally associated with the appearance of oral herpes lesions.
HSV type 2 encephalitis HSV type 2 is the main causative agent of herpes simplex encephalitis in newborns. In adults, on the other hand, this virus usually causes only a benign lymphocytic meningitis. The incidence is 1:200 to 1:5000 births. Neonatal infection is acquired during delivery by direct contact with maternal genital herpes lesions. The risk of infection in a mother with a primary genital infection, at 30 to 50%, is much greater than in women with a recurrent genital infection (maximum risk of 3%). Intrauterine infection is rare.
Clinical manifestations and treatment A primary HSV type 1 infection is asymptomatic in most cases. Infection of the oropharyngeal mucosa runs a 2- to 3-week course marked by sore throat or gingivostomatitis. Occasionally, mucosal involvement is also manifested by fever and dysphagia. The prodromal stage of encephalitis presents with flulike symptoms lasting 1 to 4 days. Most patients with HSV type 1 encephalitis have fever and hemicranial or holocephalic headaches accompanied by confusion and altered mental status. Aphasia, seizures, and hemiparesis are typical focal neurologic deficits. Immunocompromised patients are more likely to experience a less acute, relapsing and remitting course. Concomitant oral herpes is rare in these patients. CSF findings include a lymphocytic pleocytosis of 20 to 300 cells/μL. In over 80% of cases the CSF protein is elevated above 0.5 to 2.6 g/L. HSV encephalitis can be diagnosed when HSV-specific antibody titers in the CSF are elevated several-fold above baseline 2 weeks after symptom onset. The direct detection of viral DNA by the polymerase chain reaction (PCR) provides the fastest and most reliable test for establishing the diagnosis. Besides the clinical, radiologic, and CSF findings, HSV encephalitis also produces typical electroencephalograph (EEG) changes. The mainstays of treatment are general supportive measures and specific therapy with aciclovir, which can reduce the mortality of the disease in adults from 70% (untreated) to less than 20%. Mortality in children can be lowered from 80% to 50%. Other effective virostatics are famiciclovir and valacyclovir, but no studies have yet been published on these drugs.
Pathology The pathologic correlate of herpes encephalitis is a fulminant, hemorrhagic, necrotizing meningoencephalitis with a predilection for the limbic system. The gross pathology of HSV type 1 encephalitis consists of bilateral, predominantly temporobasal and frontobasal necrotic lesions. These changes are more diffuse in HSV type 2 encephalitis. Microscopy reveals perivascular infiltrates, gliotic changes, destruction of nerve cells, and viral inclusion bodies. An inflammatory reaction may initially be absent in immunocompromised patients despite CNS involvement. In patients with aquired immune deficiency syndrome (AIDS), for example, typical hemorrhagic encephalitis is not present in advanced stages. Instead there are massive viral inclusions in the affected cells, so that most neuronal damage results from direct toxic effects of the virus. Based on current practice standards, brain biopsy is no longer necessary in the diagnosis of herpes encephalitis.
MRI findings
HSV type 1 encephalitis Unlike other viral encephalitides, this disease has very specific MRI features: Focal areas of brain edema appear on PDw, T2w, and FLAIR images as hyperintensities located in the medial and lower temporal lobe and extending into the insula and cingulate gyrus. The putamen is typically spared. These signs are consistently detectable by at least 48 hours after onset of clinical symptoms. The value of advanced MRI techniques in the early diagnosis of HSV type 1 encephalitis, such as DWI, has not yet been adequately tested. In our experience, the initial cell damage in some patients may be manifested temporarily as cytotoxic brain edema with a decreased ADC. Enhancement is usually absent in the early phase of the disease. Later images show meningeal enhancement and gyriform enhancement at the gray–white matter junction ( ▶ Fig. 5.4). It usually takes several days for parenchymal petechial hemorrhages to appear; they are typically located at the gray–white matter junction and show initial high T1w signal intensity (methemaglobin). In addition to standard sequences, an optimum MRI protocol should include coronal T2w or FLAIR images and contrast-enhanced images. Serial scans over a period of days to weeks will show involvement of the contralateral hemisphere with the same sites of predilection. Resolution of the inflammation usually leaves residual cystic–gliotic changes with focal or diffuse brain atrophy. Some cases may also show dystrophic calcifications that are best demonstrated by CT scans, T2*w images, or SWI. Also, as in other inflammations, MR spectroscopy (MRS) shows a fall in the ratio of N-acetylaspartate to phosphocreatine and a rise in the ratio of choline to phosphocreatine.

Fig. 5.4 HSV type 1 encephalitis in various patients. The hyperintensities in the left temporal lobe (a–d) on T2w and FLAIR images represent inflammatory brain edema. A typical finding is the involvement of limbic structures such as the insular cortex (a–d), contralateral temporal lobe (c), and the cingulate gyri on both sides (a,b). Besides brain edema, the only sign of meningeal involvement by viral infection in the early stage is leptomeningeal enhancement (e). Typical scalloped pattern of gyral (corticomedullary) enhancement is not seen until later stages (e). The high signal intensity at the gray–white matter junction in (f) reflects a combination of contrast enhancement and hemorrhagic transformation of the brain parenchyma (panels c–e courtesy of Prof. A. Dörfler, Department of Neuroradiology, Erlangen University Hospital).
(a) Axial T2w image. (b) Axial T2w image (different plane). (c) Coronal T2w image. (d) Axial FLAIR image. (e) Axial T1w image after contrast administration. (f) Axial T1w image after contrast administration (different plane).
Note
Herpes encephalitis is a viral encephalitis that displays typical features on MRI.
HSV type 2 encephalitis The imaging features of this disease are characterized by nonspecific brain swelling with brain edema and leptomeningeal enhancement. Diagnosis is hampered by the fact that the neonatal brain is largely unmyelinated, resulting in increased signal intensity of the white matter on T2w images. Hemorrhagic transformation may occasionally occur as in HSV type 1 encephalitis.
5.2.1.2 Cytomegalovirus Encephalitis
Epidemiology CMV encephalitis in immunocompromised adults (e.g., people with AIDS) results from reactivation of the virus. On the other hand, CMV encephalitis has become less common in this group of patients since the advent of highly active antiretroviral therapies (HAART). CMV encephalitis ranks ahead of toxoplasmosis, rubella, and HSV type 2 encephalitis as the most common congenital CNS infection. Approximately 1% of all newborns in the United States are affected, and 50 to 80% of women of childbearing age are seropositive for CMV. Approximately 5% of all pregnant women excrete CMV in the urine, with transmission occurring by the transplacental route. More than 60% of affected newborns have involvement of multiple organ systems including the CNS.
Clinical manifestations and treatment Fever, personality changes, and cognitive deficits are typical in the adult form. Most newborns with CMV encephalitis are asymptomatic at birth. Over time the children develop hearing loss, seizures, mental retardation, chorioretinitis, microcephaly, optic neuritis, and ocular malformations. Antiviral drugs of proven efficacy—ganciclovir, valganciclovir, and foscarnet—are available for the treatment of CMV.
Pathology CMV in newborns has a marked affinity for the subependymal germinal matrix, resulting in extensive periventricular tissue necrosis. Eosinophilic inclusion bodies are found on histologic examination.
MRI findings T2w images in the adult form of CMV encephalitis show hyperintensities that are located mainly in the periventricular and subependymal white matter, have indistinct margins, and produce a slight mass effect. Unenhanced image findings may be mistaken for HIV encephalitis. Because the changes in CMV encephalitis are usually associated with subependymal enhancement while the inflammatory changes of HIV are not, this can provide a useful differentiating criterion. Typical findings in neonates and children are microcephaly, brain atrophy with enlarged CSF spaces, migrational abnormalities, and delayed myelination. CT is better than MRI for detecting periventricular calcifications in the neonatal form. Complicating ventriculitis may be manifested by ependymal enhancement with hydrocephalus.
5.2.1.3 Epstein–Barr Virus Encephalitis
Epidemiology The primary infection is infectious mononucleosis. Encephalitis or a Guillain–Barré syndrome may develop during the course of the primary infection or after viral reactivation. In the interval, EBV infection is associated with Burkitt’s lymphoma and nasopharyngeal carcinoma. Serologic studies show that 90% of the general population have had an EBV infection by 40 years of age.
Clinical manifestations and treatment The clinical findings include stiff neck, cranial nerve deficits (e.g., Bell’s palsy), Guillain–Barré syndrome, and seizures. Standard therapeutic agents are aciclovir and ganciclovir.
MRI findings Involvement of the basal ganglia is usually observed. EBV cerebellitis has been described.
5.2.1.4 Varicella Zoster Virus Encephalitis
Epidemiology The incidence is 1 to 2:10,000 population per year. The primary infection is chickenpox. Reactivation of the virus leads to herpes zoster (shingles) or infectious encephalitis. Other CNS manifestations are para- or postinfectious encephalomyelitis or cerebellitis following a chickenpox infection in 1:4000 children, Guillain–Barré syndrome, or Reye’s syndrome. Most patients with VZV encephalitis have a prior history of shingles. The risk is increased in immunocompromised patients.
Clinical manifestations and treatment Typical clinical manifestations are headache, malaise, and confusion occurring days to months (average 9 days) after the appearance of a shingles rash, although encephalitis may also develop with no prior history of shingles. The standard specific therapeutic agent is aciclovir, and corticosteroids are added if there is associated vasculitis. More modern drugs such as valacyclovir and famiciclovir are effective for herpes zoster but have not yet been tested for treatment of VZV encephalitis. The mortality rate of encephalomyelitis following a chickenpox infection is 30%, compared with only 0 to 5% for cerebellitis, depending on the study. The disease has a good prognosis in adults.
Pathology Various cell types are affected: oligodendrocytes, ependymal cells, and endothelial cells. Histology reveals purely inflammatory changes, hemorrhagic necrosis, and angiitic changes. These may result in secondary ischemic (vasculitic) brain infarcts. In the postinfectious form, it is believed that direct viral-mediated tissue damage is compounded by secondary immune-mediated mechanisms. Intrauterine infection leads to a severe, necrotizing encephalomyelitis.
MRI findings Three patterns of involvement are typically found in immunocompromised patients:
Hemorrhagic or nonhemorrhagic brain infarcts in the territories of large or medium-sized arteries.
Ischemic or demyelinating lesions in the deep white matter due to small-vessel involvement.
Ventriculitis or periventriculitis.
Accordingly, T2w images show multiple, often spherical white-matter lesions with a predilection for the periventricular region, cerebellum, and cervical cord. Cortical and subcortical infarcts due to vasculitis are often found in the distribution of small- and large-caliber vessels, with some areas showing hemorrhagic transformation. In cases that develop ventriculitis or periventriculitis with periventricular T2w hyperintensity and homogeneous enhancement, the findings may resemble those of CMV encephalitis. Contrast enhancement may occur. MRA may demonstrate abrupt caliber changes in the cerebral vessels.
5.2.1.5 Progressive Multifocal Leukoencephalopathy
Epidemiology The causative organism of PML is the JC virus, a DNA-containing polyomavirus named after the initials of the first reported patient. PML develops in 4 to 7% of all AIDS patients, with a rising trend in recent years. This makes PML the most common opportunistic viral infection of the CNS. The risk of developing PML is also increased in all other diseases with a severe cellular immune defect such as the leukemias, Hodgkin’s disease, and autoimmune disorders. There have been reports in recent years of diseases besides classic PML that are also linked to the JC virus:
PML may develop in response to potent immunosuppressant therapy with monoclonal antibodies such as natalizumab, efalizumab, and rituximab, which are used in the treatment of multiple sclerosis, Crohn’s disease, psoriasis, and hematologic and rheumatic diseases.
PML- ▶ IRIS (immune reconstitution inflammatory syndrome) results from an exaggerated immune response to antigens of opportunistic pathogens like the JC virus. IRIS may arise during the treatment of HIV-positive patients with HAART, marked by a rapid increase in CD4 cells and a rapid decline in the viral burden. IRIS may also result from the sudden discontinuation of potent immunosuppression with a monoclonal antibody.
Note
A PML infection may develop in response to immunomodulatory therapy. Consequently, these patients should undergo regular cranial MRI examinations so that any infection can be promptly detected.
Clinical manifestations and treatment Clinical findings may include headaches, cognitive deficits, visual field defects, or ataxia depending on the affected structure. Hemiparesis is also present in some cases. The disease is usually fatal within 1 year after diagnosis. There is no proven effective or specific drug therapy, although positive response to cidofovir has been reported. The overriding goal is to restore immunocompetence by discontinuing immunosuppressant therapy at once and, if the underlying disease is AIDS, instituting optimum antiretroviral therapy.
Pathology The JC virus infects and destroys oligodendrocytes, resulting in multifocal demyelination.
MRI findings The most common site of involvement by classic PML is the posterior centrum semiovale. Typically the disease begins in the posterior, subcortical lobar white matter. The U-fibers are always involved; this differs from the pattern in HIV encephalopathy and is a useful differentiating feature ( ▶ Fig. 5.5). The cortical gray matter is spared. Imaging in advanced stages demonstrates white-matter lesions with high T2w signal intensity that expand over time and become confluent. The thalamus, basal ganglia, corpus callosum, and infratentorial structures may also be affected. Involvement of the brainstem and cerebellum is also common. Contrast enhancement is almost always absent, and mass effect is rare. Unlike classic PML, enhancement is more likely to occur in PML treated with potent immunosuppressive monoclonal antibodies such as natalizumab ( ▶ Fig. 5.6), efalizumab, and rituximab, and also in PML-IRIS and IRIS.

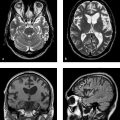
Fig. 5.5 Classic PML. Axial T2w images in a 33-year-old woman with AIDS. (a) Hyperintensities in the posterior white matter of the occipital lobes are a typical finding. In this case they are more pronounced on the left side. (b) In contrast to HIV encephalitis, the U-fibers (arcuate fibers) are also involved. Infratentorial structures may also be affected by the disease.

Fig. 5.6 Atypical PML in a multiple sclerosis patient treated with natalizumab. MRI shows patchy FLAIR hyperintensities in the frontal white matter and right occipital lobe with a slight mass effect (a) and contrast enhancement (b). Enhancement is not typically present in classic PML but may occur during treatment with a monoclonal antibody, as in this case.
(a) Axial FLAIR image. (b) Axial T1w image after contrast administration.
5.2.1.6 Congenital Rubella Encephalitis
Epidemiology The rubella virus is transmitted from the maternal bloodstream to the fetus by the transplacental route. The severity of the disease depends on the gestational age at infection. A complete rubella syndrome develops when infection occurs in the first trimester. The disease has become rare in developed countries owing to widespread rubella immunization.
Clinical manifestations and treatment Infection may result in spontaneous abortion, stillbirth, or severe fetal damage, depending on the timing of the infection. Besides encephalitis, rubella syndrome may include ocular and cardiac malformations. Deafness is the most important late symptom.
Pathology The virus interferes with cellular division and maturation, especially of progenitor cells in the germinal matrix. Histologic examination shows inflammatory infiltration of the meninges and perivascular spaces.
MRI findings Typical findings are microcephaly and parenchymal calcifications, predominantly in the basal ganglia.
5.2.1.7 Tick-Borne Encephalitis
Epidemiology The incidence of tick-borne encephalitis (TBE) is approximately 1 to 2:100,000 population per year. The causative organisms are arboviruses (flaviviruses), with small wild rodents as their principal reservoir. Generally the virus is transmitted to humans by tick or mosquito bites. Most outbreaks occur in June or July, accounting for the alternate name for the disease, “early summer meningoencephalitis.” Approximately 0.1% of ticks are infected with the virus. Workers in agriculture and forestry are at highest risk.
Clinical manifestations and treatment Sixty percent of infected individuals have no symptoms, and 30% develop flulike symptoms within the first 3 weeks. Only 10% of patients develop meningoencephalitis following a latent period of 1 week. The encephalitis presents with high fever of acute onset and organic psychosis. Cranial nerve deficits, extrapyramidal motor hyperkinesia, cerebellar signs, and paralysis may also occur. Children usually develop only a benign lymphocytic meningitis and have a better prognosis than adults. Involvement of autonomic brainstem centers implies a poor prognosis. Overall mortality rate is 1%. An antiviral therapy is not available. Inoculation with polyvalent active vaccine is recommended for people visiting or living in endemic areas.
Pathology The pathologic findings are nonspecific.
MRI findings MRI findings are normal in the majority of cases. Possible abnormalities consist of T2w hyperintensities in the thalamus, cerebellum, brainstem, and caudate nucleus ( ▶ Fig. 5.7).

Fig. 5.7 FSME in a 24-year-old man. Axial FLAIR images show hyperintensities in the cerebral structures displayed in (a–d).
(a) Upper right medulla oblongata. (b) Upper right medulla oblongata (different plane). (c) Left centrum semiovale. (d) Right midbrain.
5.2.1.8 Rabies Encephalitis
Epidemiology Rabies encephalitis is an acute infection involving the CNS in humans and other mammals. It has a worldwide distribution and only a few countries are free of rabies, including the United Kingdom, Japan, Australia, and New Zealand. The causative agent is a cylindrically shaped RNA virus of the rhabdovirus family. The disease is most commonly transmitted to humans through the bite of infected dogs, cats, foxes, hedgehogs, wolves, and vampire bats. Cattle, horses, small game, and other herbivores may be infected but rarely transmit the virus to humans. Following a bite from an infected animal, the virus travels along the axons to the CNS, where it replicates. The incubation period is 3 to 10 weeks in most cases but may last for years. The closer the portal of entry (bite wound) to the brain, the shorter the incubation period. The virus spreads hematogenously from the CNS to other organs. Approximately 20 to 50% of people who are bitten actually develop rabies. The estimated worldwide death toll from rabies is at least 40,000 to 70,000 annually. Approximately 10 million people undergo preventive treatment after contact with animals suspected of having rabies.
Clinical manifestations and treatment The disease progresses in several stages:
Stage 1 is marked by initial nonspecific symptoms such as fever, headache, nausea, vomiting, abdominal pain, diarrhea, and cough. Later signs include irritability and hypersensitivity to light, noise, and drafts, accompanied by a steadily rising fever.
Stage 2, the acute neurologic stage or excitation stage, is characterized by hyperactivity with muscle twitches and spasms. Associated signs are anxiety, agitation, alternating aggressive and depressive mood, and hydrophobia. The visual or acoustic perception of water triggers agitation and seizures, which may spread to all the muscles. In 80% of cases this stage is an encephalitic stage (“furious rabies”), while the remaining 20% experience a paralytic form (“dumb rabies”). This phase lasts for 2 to 7 days. Finally the patient lapses into a coma, followed by death due to respiratory paralysis or central circulatory failure. Rabies is almost invariably fatal once initial symptoms have appeared.
Consequently, treatment must be instituted quickly after the bite from a suspicious animal. Intensive care is the only option available for prolonging survival in patients who become symptomatic. In addition to local wound cleansing and debridement, the area around the injury site should be infiltrated with anti-rabies hyperimmunoglobulin. This agent should also be injected intramuscularly, possibly in combination with interferon. Dead vaccine should be administered on the following day. Prophylactic vaccination is advised in persons at high occupational risk (hunters, skinners, veterinarians and veterinary nurses, laboratory staff, and travelers to certain countries in Asia or Africa).
Pathology Macroscopically, the brain tissue shows multifocal areas of hemorrhage, necrosis, and edema in the gray matter, especially the basal ganglia, and also in the brainstem. Histology shows axonal necrosis with degeneration of axis cylinders and demyelination. Seventy percent of rabies cases are associated with the formation of cytoplasmic inclusions called Negri bodies. They are most abundant in the pyramid cells of the hippocampus and cerebral cortex and in the Purkinje cells of the cerebellar cortex.
MRI findings Only a few case reports have been published on radiologic findings. Rabies shows a certain initial predilection for infection of the gray matter. T2w images may show diffuse hyperintensities in the basal ganglia, thalamic pulvinar, periventricular white matter, hippocampus, and brainstem. Diffuse brain edema develops in advanced cases. Hyperintensities on T1w images suggest hemorrhagic transformation (extracellular methemaglobin). Vasculitis-like changes with vascular stenoses and brain infarcts have also been described.
5.2.1.9 Measles
Epidemiology The following forms of encephalitis may develop as a sequel to measles infection:
Acute measles encephalitis: Before the advent of childhood vaccination, the incidence of measles encephalitis was 10:100,000 children under age 16. It began shortly after appearance of the measles rash or several months later as an acute progressive encephalitis. The likelihood of developing encephalitis after measles vaccination is 1.8:100,000 vaccinations.
Subacute measles encephalitis: This form is typically manifested 1 to 10 months after a measles infection in immunocompetent patients.
Subacute sclerosing panencephalitis (SSPE): This form occurs months to years (average of 7 years) after a measles infection and predominantly affects children and adolescents who had clinical measles before the age of 2. It usually develops before 30 years of age, with average onset occurring at age 7. It is caused by a mutation of the measles virus. The annual incidence is 1:1,000,000 in the nonimmunized population.
Clinical manifestations and treatment
Acute measles encephalitis Acute encephalitis is characterized by fever and seizures. Postinfectious or autoimmune encephalitis occurs during convalescence from measles and is typically marked by a recurrence of fever and impaired consciousness, seizures, and focal neurologic deficits days or several weeks after the primary infection.
Subacute measles encephalitis The clinical manifestations of subacute measles encephalitis include refractory seizures. Fever is not present.
SSPE SSPE is characterized by myoclonus, epileptic seizures, and altered mental status. Periodic EEG complexes accompany the initial (psychopathologic) stage and subsequent neurologic stage. There is no known definitive treatment, but the disease appears to respond favorably to early initiation of combination therapy with high-dose intrathecal α–interferon plus intravenous ribavirin.
Pathology Histopathology in SSPE shows nerve cell damage, astrogliosis, foci of demyelination, and inflammatory infiltration. Intranuclear inclusions are present in neurons, oligodendrocytes, and astrocytes, which are found to contain nucleocapsids on electron microscopy.
MRI findings
Acute measles encephalitis T2w images in acute encephalitis show hyperintense lesions in the subcortical white matter, brainstem, and cerebellum.
SSPE Initial findings in SSPE are usually normal. Imaging later in the disease shows diffuse brain swelling and the appearance of focal T2w hyperintensities in the white matter, cortex, basal ganglia (especially the putamen and caudate nucleus), and cerebellum. Gross brain atrophy eventually develops.
5.2.1.10 HIV Encephalitis and Encephalopathy
Epidemiology The terms “HIV encephalitis” and “HIV encephalopathy” are used interchangeably in the literature. HIV encephalitis or encephalopathy develops in 60% of people with AIDS and is caused directly by the HIV organism. HIV encephalitis or encephalopathy is the earliest manifestation of the disease in 3–10% of all people with AIDS. Besides HIV encephalitis, a number of other opportunistic infections may occur in this immunocompromised population:
Toxoplasmosis.
JC virus infection (PML).
Tuberculosis.
Neurosyphilis.
VZV encephalitis.
Herpes encephalitis.
CMV encephalitis.
Cryptococcosis.
Clinical manifestations and treatment The disease presents clinically with slowly progressive dementia, fatigue, apathy, and inattention. Later symptoms often include disturbances of motor coordination such as tremor and altered gait. Children with HIV encephalitis manifest developmental delay, behavioral disorders, and motor deficits. No specific treatment is available, but deficits may improve in response to HAART. HAART regimens are constantly expanding as new medications are added with the goal of suppressing the viral burden with the fewest possible side effects.
Pathology Involvement of the CNS by the virus incites an inflammatory parenchymal response with the formation of multinucleated giant cells and glial nodules. Over time the infection leads to demyelination, loss of axons, and brain atrophy. Histopathology may reveal cortical involvement before any imaging signs of this involvement appear. Damage to the limbic system and corpus striatum is attributed to the toxic effect of quinolinic acid, which is released by activated macrophages and microglial cells in response to HIV involvement.
MRI findings As in other encephalitides, the neuroimaging findings of HIV encephalitis are nonspecific:
In the first stage, multiple white-matter hyperintensities approximately 1 cm in size can be seen on T2w images in 20% of patients, usually at the time of initial HIV infection. The lesions are usually symmetrical and form confluent patches. The cortex and U-fibers are spared; this allows differentiation from PML, which always involves the U-fibers. Magnetization transfer (MT) measurements may also be helpful in this respect: While lesions in HIV encephalitis show only about a 20% decline in the MT ratio relative to normal values, the MT ratio in PML is decreased by approximately 45% due to greater demyelination. Usually the lesions do not show contrast enhancement.
The second stage of HIV encephalitis is characterized by a progressive subacute encephalitis with brain atrophy. Central atrophy (ventricular enlargement) usually predominates over cortical atrophy (sulcal widening). The brain atrophy is gradually progressive over a period of months. In time, calcifications may appear in the basal ganglia, frontal lobes, and cerebellum. HIV encephalitis in children may also be associated with vasculitis and aneurysm formation.
A variant of HIV encephalopathy is progressive diffuse leukoencephalopathy (PDL). The signal changes in this condition are found chiefly in the frontal white matter and always spare the U-fibers.
5.2.1.11 Other, Less Common Viral Encephalitides
Half of all encephalitides in Sweden are caused by tick-borne virus.
Japanese B encephalitis, the most common encephalitis in Asia, is caused by a virus belonging to the St. Louis complex of flaviviruses. The disease is epidemic in China, the northern portions of Southeast Asia, and portions of India and Sri Lanka. The virus is transmitted by mosquitoes. Children are most commonly affected, and nonimmune adults who travel to an epidemic region may develop a severe encephalitis. The infection may be asymptomatic or may cause simple flulike symptoms. Encephalitis is characterized by high fever, headaches, seizures, and coma. Tremor and other parkinsonian symptoms may also occur. The virus affects the brainstem nuclei, basal ganglia, and thalamus.
The lymphocytic choriomeningitis virus (belonging to the group of arenaviruses) is transmitted by mice and hamsters that are kept as pets. Infection may incite a meningitis with mild accompanying encephalitis.
Encephalitis caused by paramyxoviruses like the parainfluenza virus is also rare.
5.2.1.12 Differential Diagnosis
In principle, the differential diagnosis should include nonviral encephalitides, which may closely resemble the viral encephalitides in some cases ( ▶ Table 5.3). Besides excluding complications such as brainstem involvement, the main task of the neuroradiologist is to differentiate inflammatory changes from neoplastic, paraneoplastic, or immunologic diseases and ischemic changes. Especially in patients with a relapsing–remitting or slowly progressive clinical course, which is common in immunocompromised patients, the morphologic imaging features may sometimes be consistent with a low-grade primary brain tumor if only T1w and T2w sequences are obtained. A change in enhancement characteristics within days in viral encephalitis; hemorrhagic transformation, which is usually absent with low-grade gliomas; and the rapid spread of inflammatory changes (e.g., in limbic structures) like that occurring in HSV type 1 encephalitis are signs that may help to narrow the differential diagnosis. On perfusion MRI of higher-grade gliomas and metastases, unlike inflammatory changes, the regional cerebral blood volume will be at least twice the volume in normal brain tissue. Lymphomas and foci of toxoplasmosis, which are the most important entities requiring differentiation from HIV-associated diseases, always show contrast enhancement. Lymphomas always cause massive disruption of the blood–brain barrier, which usually outweighs the T2*w effect from the (slightly) increased regional cerebral blood volume on perfusion MRI.
Bacteria | Parasites | Fungi | Rickettsiae |
|
|
|
|
All brain infarcts initially show a decreased ADC, conform to vascular territories, and show more rapid demarcation than inflammatory foci. Brain infarcts have such typical stage-dependent MRI characteristics that they are usually distinguishable from viral encephalitis—if not immediately then on analysis of follow-up images. The differential diagnosis of VZV encephalitis also includes cerebral vasculitis due to various causes. Rabies encephalitis may resemble a number of other diseases on neuroimaging due to frequent involvement of the basal ganglia, thalamus, and brainstem:
Spongiform encephalopathies.
Wilson’s disease.
Leigh’s disease.
Toxic encephalopathies from exposure to carbon monoxide, methanol, cyanides, or hydrogen sulfide.
Hypoglycemia.
Hemolytic–uremic syndrome.
Definitive viral identification can be accomplished by the enzyme-linked immunosorbent assay (ELISA) technique. IgM antibodies (antibodies against immunoglobulin M) should be present for the diagnosis of acute disease. PCR is a modern, highly sensitive test for identifying viruses of the herpes group (HSV type 1, HSV type 2, CMV, VZV, EBV) and, increasingly, many other viruses based on the direct detection of viral DNA in the CSF. This test allows for early diagnosis at a time when MRI is often still negative.
In some cases it may be difficult to distinguish an acute viral encephalitis from a postinfectious acute disseminated encephalomyelitis (ADEM). Differentiating criteria are listed in ▶ Table 5.4.
Note
The main role of MRI in patients with suspected encephalitis is not to identify the causative organism but to exclude other diagnoses such as tumors and infarctions.
Findings | Infectious (viral) encephalitis | ADEM |
MRI findings | Patchy white matter hyperintensities on T2w images, often with involvement of cerebral cortex, basal ganglia, brainstem, and cerebellum Enhancement, if present, is usually homogeneous (no ring enhancement) | Multifocal, rounded white matter hyperintensities on T2w images, gray matter usually not involved Target pattern with ring enhancement All lesions at same stage of activity Possible involvement of spinal cord |
CSF findings | Lymphocytic pleocytosis, proteins elevated, glucose normal In HIV encephalitis: possible detection of red cells | Lymphocytic pleocytosis, proteins elevated, glucose normal In acute hemorrhagic encephalomyelitis: possible detection of red cells |
Days or weeks after vaccination | No | Yes |
Typical age | No age predilection | Children |
Fever | Usually present | Possible |
Prodromal complaints | Occasional | Usually present |
Unilateral or bilateral vision loss | Rare | Possible |
Spinal cord signs | Rare | Possible |
5.2.2 Bacterial Infections
5.2.2.1 Pyogenic Cerebritis and Bacterial Brain Abscess
Cerebritis is the earliest stage of a pyogenic brain infection and generally culminates in the formation of a brain abscess. The main task of neuroimaging is to differentiate cerebritis and brain abscess from ring-enhancing lesions such as metastases and brain tumors ( ▶ Table 5.5).
Imaging findings | Bacterial brain abscess | Cystic–necrotic anaplastic glioma/ glioblastoma/ metastases | ADEM or acute multiple sclerosis | Primary CNS lymphoma in immunosuppressed patients | Toxoplasmosis |
T2w images | Cyst contents hypo-, iso- or hyperintense depending on protein content Hypo- to isointense rim (capsule) Marked perifocal hyperintensity (edema) | Cyst contents usually hyperintense No continuous rim Marked perifocal edema directed toward the ventricle (glioma) | Multiple white matter hyperintensities Possible target pattern | Solidary lesion, usually larger than 1 cm × 1 cm Inhomogeneous signal: hypointense in solid portions, mostly hyperintense in cystic portions, which are more common in immunocompromised patients Possible intralesional hemorrhage | Multiple areas with hyperintense centers and marked perifocal edema in white and gray matter (basal ganglia) |
T1w images (without contrast) | Cyst contents hypo-, iso- or hyperintense depending on protein content Isointense rim (capsule) Marked perifocal hypointensity (edema) | Cyst contents usually hypointense No peripheral rim Perifocal hypointensity (edema) | Rare hyperintensity (hemorrhage) | Inhomogeneous, predominantly hypointense signal Possible intralesional hemorrhage | Multiple hypointense areas Hyperintensity due to calcification (or rare intralesional hemorrhage) of toxoplasmosis foci |
T1w images with contrast | Smooth, intensely enhancing rim that is thicker on the brain surface than near the ventricle Ring grows by c. 1 mm/month | Intensely enhancing rim, often with indistinct outer and inner margins Grows toward the ventricle | All lesions show uniform activity (contrast enhancement) or no activity (no contrast enhancement) | Ring enhancement is unusual Enhancement is inhomogeneous with solid portions showing intense enhancement Subependymal enhancement Encasement of ventricles | Multiple enhancing foci in the white and gray matter Predilection for basal ganglia |
DWI | ADC is usually decreased in cystic portion | ADC is usually increased in cystic portion | ADC may be initially decreased in subacute stage, always increased thereafter | ADC usually decreased in solid portion | ADC variable, often increased |
Perfusion MRI | Relative regional cerebral blood volume in solid portion not increased over normal brain tissue, or increased by no more than 50% | Relative regional cerebral blood volume in solid portion increased by 100% or more | Relative regional cerebral blood volume in solid portion increased by no more than 50% over normal brain tissue | Massive blood–brain barrier disruption in solid portion | Relative regional cerebral blood volume in solid portion not increased over normal brain tissue, or increased by no more than 50% |
MRS: | |||||
N-acetyl aspartate | Ø | ↓ (glioma) | ↓–↓↓ | ↓ | Ø–↓ |
Ø–↓(metastasis) | |||||
Choline | ↓–Ø | ↑ | ↑ | ↑↑ | ↑ |
Lipids | ± | ↑–↑↑↑(glioma) in necrotic portion | ± | ↑↑↑in solid portion | ↑↑ |
±–↑(metastasis) | |||||
Lactate | ↑ | ↑ | ± | ±–↑ | ↑ |
Amino acids | ↑ (only if untreated) | Ø | Ø | Ø | Ø |
PET | Metabolically active in FDG PET | Metabolically inactive in FDG PET | |||
SPECT | Usually no thallium-201 uptake | Positive thallium-201 uptake | Positive thallium-201 uptake | Usually no thallium-201 uptake | |
Other | MT ratio usually decreased by more than 45% after infection or vaccination | CSF findings positive for EBV | CSF findings positive for toxoplasmosis | ||
CSF, = cerebrospinal fluid; EBV= Epstein–Barr virus; FDG = fluorodeoxyglucose; PET = positron emission tomography; SPECT= single-photon emission computed tomography. Ø= not detectable ↑ (↓) = slightly increased (decreased) ↑↑ (↓↓) = moderately increased (decreased); ↑↑↑ (↓↓↓) = greatly increased (decreased) ± = may be present | |||||
Epidemiology The yearly incidence of brain abscesses declined from approximately 3:100,000 population in the early 20th century to less than 1:100,000 population by the end of the 20th century. Males are affected 2 to 3 times more often than females. The disease most commonly occurs in the third and fourth decades. One-third of patients are found to have more than one infecting organism. The risk is increased in immunocompromised patients such as people with AIDS and bone marrow or organ-transplant recipients. Without prophylaxis, 1 in 50 patients receiving a bone marrow transplant will develop a brain abscess. The infectious agent may reach the brain by various routes: One-half of all brain abscesses result from contiguous spread of an adjacent infection, usually sinusitis or otitis. In 10% of cases the source is a molar dental abscess. The most likely causative mechanism in this type of spread is a retrograde infectious thrombophlebitis. Twenty-five percent of all brain abscesses are caused by hematogenous spread from an extracranial focus such as bacterial endocarditis or purulent pneumonia (septic focal encephalitis). Other potential sources are infectious foci in the abdomen or pelvic cavity and osteomyelitis. The extracranial septic focus can be identified in 10 to 63% of cases. Congenital cyanotic heart disease, pulmonary vascular anomalies, and other chronic conditions increase the risk of hematogenous spread to the CNS. Bacterial meningitis in newborns may also lead to the development of a brain abscess. The most common pathogens in these cases are gram-negative organisms.
Clinical manifestations and treatment Less than 50% of patients present the classic triad of fever, headaches, and focal neurologic deficits, depending on the location of the inflammatory focus. Clinical complaints are often nonspecific and may include impaired consciousness, cranial nerve deficits, etc., especially in transplant patients. Treatment typically consists of antibiotics plus neurosurgical drainage of the abscess. As a general rule, all abscesses larger than 2.5 cm in diameter or causing a mass effect should be drained. If abscess drainage is planned, antibiotic therapy should be postponed until after the procedure so that treatment can be directed by sensitivity results. Patients with concomitant meningitis, ependymitis, hydrocephalus, or risk factors for surgery should be selected for primary antibiotic therapy alone. Blood cultures should be obtained before initiating treatment. Antibiotic selection is based on the result of microbiologic testing. If the organism is not identified, an empirical combination therapy is recommended (third-generation cephalosporins + metronidazole + an antibiotic active against staphylococci). Adjuvant corticosteroid therapy may also be advised. Treatment is usually continued for 6 to 8 weeks. Before the advent of antibiotics, the mortality rate of brain abscess was 100%. Today it is still in the range of 8 to 10%. The current mortality rate of brain abscess in organ and bone marrow transplant recipients and other immunocompromised patients is 80 to 90%. The most frequent long-term complication of brain abscess is epilepsy.
Pathology The causative organisms in 70% of cases are streptococcal species, especially Streptococcus milleri. Staphylococcus aureus is present in 10 to 30% of cases, usually after neurosurgical procedures or penetrating head injuries such as gunshot wounds. Gram-negative bacteria are found in 10 to 20% of patients. Bacteroides and Fusobacterium species are the most common anaerobes. In organ and bone marrow transplant recipients fungi such as Aspergillus and Candida are more often detected than pyogenic bacteria.
Brain abscess evolves from cerebritis in four stages:
Early pyogenic cerebritis: Once bacteria have invaded the gray or white matter of the brain, polymorphonuclear granulocytes migrate from local capillaries to the infectious focus. The capillary endothelium becomes edematous, resulting in early pyogenic cerebritis without a capsule. The cerebritis is focal but not yet localized. Pathology shows scattered necrotic changes, edema, hyperemia, and perivascular inflammatory infiltrates. This stage lasts approximately from days 3 to 5.
Late cerebritis: In this second stage (from 4–14 days), smaller necrotic zones coalesce to larger zones and the infection becomes more localized. Pathology shows vascular proliferation with an accumulation of inflammatory cells, macrophages, granulation tissue, and fibroblasts around the inflammatory focus.
Early capsular stage: This stage begins at approximately 2 weeks postinfection. Collagen and reticulin fibers form an abscess capsule.
Late capsular stage: This stage may persist for weeks or months. A complete capsule is formed, consisting of an inner layer with inflammatory cells, an intermediate collagen layer, and an outer gliotic layer. The capsule is thicker on the cortical side of the abscess than on the medial side. This relates to the specific vascular architecture of the brain.
The time course of this evolution from cerebritis to a mature abscess is variable and depends on the patient’s immune status and the start of antibiotic therapy.
MRI findings
Stages of abscess development Cerebritis and brain abscess are most commonly located at the gray–white matter junction of a cerebral hemisphere, usually in the frontal or parietal lobe. Fewer than 15% of all brain abscesses occur in the posterior fossa. Multiple abscesses are particularly common in immunocompromised patients. Early cerebritis appears as focal but ill-defined brain edema with hyperintensity on T2w and FLAIR images. Contrast enhancement may be present or absent; when present, it is often blurry and indistinct ( ▶ Fig. 5.8). In the late cerebritis stage, necrotic zones form increasingly well-defined areas of CSF intensity within the edema. A peripheral rim that is iso- to slightly hyperintense on T1w images and iso- to slightly hypointense on T2w and PDw images may already be present as evidence of the developing abscess capsule. Hemorrhagic areas are usually present in septic focal encephalitis ( ▶ Fig. 5.9). Although FLAIR imaging is the more modern technique and has largely replaced “classic” double-echo imaging in tumor diagnosis, for example, T2w and PDw images are still better for detecting the very specific hypointensity of the abscess capsule. The peripheral rim (abscess capsule) shows intense, homogeneous ring enhancement and is always detectable in the early and late capsular stage ( ▶ Fig. 5.10). The thickness of the ring increases by approximately 1 mm per month. This enhancing rim is thinner on the medial side of the abscess than on the side facing the cerebral cortex. Hematogenous abscesses are usually multifocal and are distributed along the territory of the MCA (“septic focal encephalitis”). The presence of multiple, concentric ring structures a few millimeters in diameter that are hypointense on T2w images is fairly typical of abscesses caused by Nocardia asteroides ( ▶ Fig. 5.11). Patients with systemic diseases such as lymphoma or leukemia often develop multiple abscesses. The capsule will become thinner and show less enhancement in response to corticosteroid therapy. If the abscess establishes contact with the inner CSF spaces, ependymitis may develop ( ▶ Fig. 5.12).

Fig. 5.8 Cerebritis. T2w image shows increased signal intensity in the left hemisphere due to brain edema. Hyperintensities are noted in the basal ganglia, anterior thalamic nuclei, cingulate gyrus, insular cortex, and occipital lobe. An abscess capsule is not yet visible. (a) Axial T2w image. (b) Axial T1w image after contrast administration.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree