Inferior Vena Cava
Richard Gordon
Matthew Lyon
INTRODUCTION
Ultrasound evaluation of dynamic change in inferior vena cava (IVC) diameter has become useful for the diagnosis and treatment of acutely ill patients with hypotension, dehydration, and dyspnea. In the early 2000s, goal-directed therapy became the standard of care for the management of patients with septic shock. Central venous pressure (CVP) was specifically recognized as the best hemodynamic parameter to determine volume responsiveness (1, 2, 3, 4, 5). Central venous access required for CVP monitoring carries risk for complications such as arterial puncture, venous thrombosis, and infection. Not only is the procedure to obtain central venous access time consuming, but placement is often delayed until after intravenous (IV) fluid boluses fail or after elevated lactic acid is reported. Practical limitations exist as well, ranging from expensive and specialized equipment to the availability of trained personnel to monitor CVP continuously (6,7). A survey conducted in 2004 highlighted these limitations after reporting that only 7% of academic emergency departments (ED) had initiated protocols for invasive hemodynamic monitoring (8). Interest in dynamic measurement of the IVC grew as investigators began to search for a widely available, rapidly employed, cost-effective, and noninvasive means of quantifying CVP.
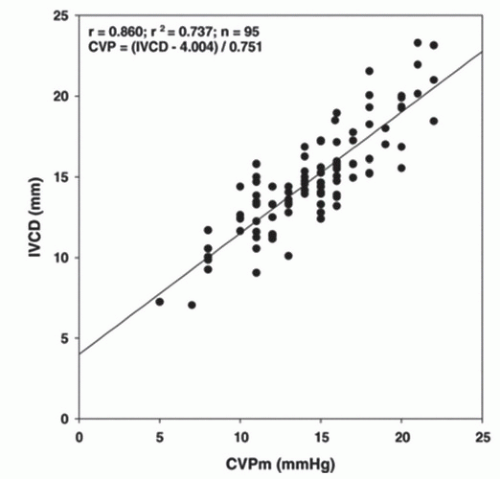
The IVC is a distensible conduit for return of blood from the inferior systemic circuit to the right heart. Because the IVC is so distensible, the diameter is dependent on an assortment of variables, including circulating blood volume, right heart pressure, intrathoracic pressure, and abdominal pressure. Since the 1970s, sonographic description of IVC caliber variation with respiratory cycling has been widely reported (9, 10, 11, 12, 13, 14). In 1990, Kircher et al. reported a noninvasive estimation of right atrial pressure by measuring IVC collapse (caval index) with end inspiration. The caval index opened the door for numerous studies correlating CVP with IVC diameter and degree of IVC respiratory collapse (Fig. 6.1) (15, 16, 17, 18, 19, 20, 21, 22, 23). Today, bedside ultrasound assessment of the IVC provides the emergency physician with a quick, inexpensive, noninvasive, valuable hemodynamic parameter that serves to narrow the differential for undifferentiated hypotension and guide initial resuscitation efforts.
CLINICAL APPLICATIONS
There are three principal clinical scenarios in which bedside ultrasound evaluation of IVC collapse is beneficial: (1) Adjunct to the clinical assessment of the undifferentiated hypotensive patient, (2) Adjunct to volume resuscitation of the volume-depleted patient, (3) Adjunct to the clinical assessment of acute dyspnea.
Undifferentiated Hypotension
The differential diagnosis for hypotension is broad with certain diagnoses requiring rapid and very specific therapy. To determine whether the patient needs large volume
resuscitation, thoracostomy, pericardiocentesis, thrombolysis, vasopressor support, inotropic support, surgical intervention, or a combination of these therapies can be extremely difficult based on clinical merits alone. The emergency physician can gain rapid insight into cardiac preload hemodynamics with bedside analysis of the IVC.
resuscitation, thoracostomy, pericardiocentesis, thrombolysis, vasopressor support, inotropic support, surgical intervention, or a combination of these therapies can be extremely difficult based on clinical merits alone. The emergency physician can gain rapid insight into cardiac preload hemodynamics with bedside analysis of the IVC.
In the face of hypotension, identification of a plethoric IVC suggests high right atrial pressure (23), prompting the physician to heavily consider diagnoses such as pulmonary embolus (PE), cardiac tamponade, tension pneumothorax, and cardiogenic shock. In contrast, visualization of a collapsible IVC suggests a low right atrial filling pressure (23), prompting the clinician sonographer to initiate volume replacement while considering underlying diagnoses for hypovolemia. Ultrasound evaluation of undifferentiated hypotension will be discussed further in Chapter 7.
TABLE 6.1 Ultrasound Appearance of the Right Ventricle, Left Ventricle, Right Atria, and Inferior Vena Cava Relative to the Different Categories of Shock | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
Guide to Volume Resuscitation
Acute care physicians frequently administer IV fluids with the goal of increasing preload and ultimately cardiac output (24). Patients who have increased cardiac output in response to an increase in preload are considered fluid responsive. On the other hand, overzealous administration of IV fluids may be deleterious, increasing the risk of Acute Respiratory Distress syndrome (ARDS), pulmonary edema, peripheral edema, cerebral edema, electrolyte abnormalities, and dilutional coagulopathy (25,26). The challenge resuscitative physicians face is how much fluid to give without giving too much. Bedside evaluation of the caval index can help identify patients who are fluid responsive at presentation (15, 16, 17, 18, 19, 20, 21, 22,27, 28, 29), while serial measurements can identify patients who remain fluid responsive despite initial fluid resuscitation (18,22).
Evaluation of Dyspnea
Emergency physicians also frequently treat patients with acute dyspnea. Differentiating illness such as obstructive pulmonary disease from decompensated congestive heart failure (CHF) is challenging because the physical exam is unreliable and no single test is adequate (30). A study of 46 patients conducted by Blehar and colleagues demonstrated the usefulness of the caval index in differentiating dyspnea from CHF and fluid overload from non-CHF causes. They reported an IVC collapse of 9.6% in the volume-overloaded patients and an IVC collapse of 46% in non-CHF patients (31). Other diagnostic considerations include patients with massive PE, pericardial tamponade, and tension pneumothorax. There are numerous echocardiographic findings that diagnose elevated right heart pressures secondary to obstructed IVC return or pulmonary outflow tract (Table 6.1) (32, 33, 34).
IMAGE ACQUISITION
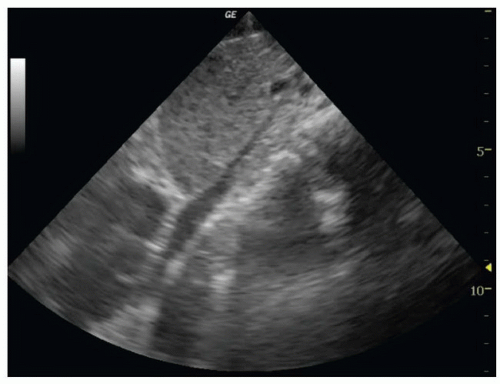
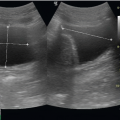
To image the IVC, place the patient in a supine position. A curvilinear 3 to 5 MHz probe or phased array 2 to 5 MHz probe provides adequate penetration to interrogate the IVC. The scan should begin in a midline sagittal orientation just below the xyphoid process with the probe indicator pointing to the patient’s head. The sonographer then slides the probe 1 to 2 cm to the patient’s right to bring the IVC into the field of view (Fig. 6.2). Slight angling of the probe to aim under the costal margin will reveal the entry point of the IVC
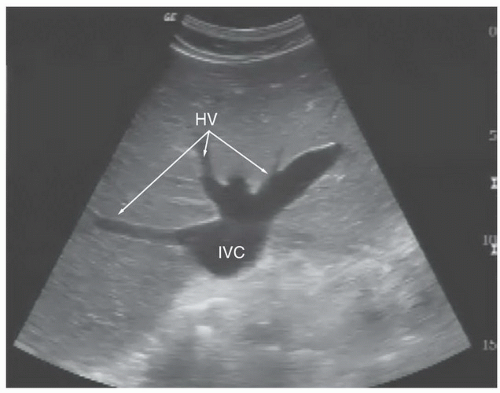
into the right atrium. Ideally, the hepatic vein inlet should be visualized entering the IVC about 1 cm caudal to the right atrium (Fig. 6.3). The hepatic vein inlet is a key point of reference because measurements of the caval index are typically conducted 1 cm caudal to this location. In volume-depleted patients the hepatic veins may not be visible secondary to complete collapse of the vessel. If this is the case, the sonographer should conduct caval index measurements 2 to 3 cm caudal to the right atrium (Fig. 6.4).
into the right atrium. Ideally, the hepatic vein inlet should be visualized entering the IVC about 1 cm caudal to the right atrium (Fig. 6.3). The hepatic vein inlet is a key point of reference because measurements of the caval index are typically conducted 1 cm caudal to this location. In volume-depleted patients the hepatic veins may not be visible secondary to complete collapse of the vessel. If this is the case, the sonographer should conduct caval index measurements 2 to 3 cm caudal to the right atrium (Fig. 6.4).
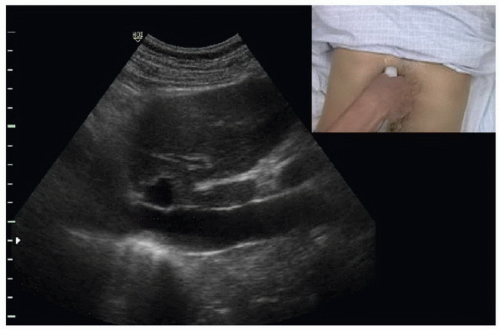 FIGURE 6.2. Long-Axis Orientation Slightly Right of the Patient’s Midline to Obtain a Long-Axis Image of the IVC. |
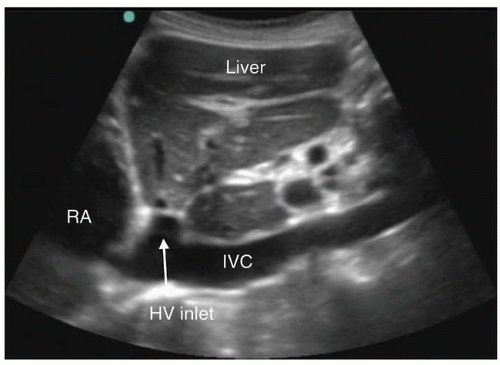 FIGURE 6.3. Long-Axis Image of the IVC Traversing the Liver to Enter the Right Atrium (RA). The hepatic vein (HV) is seen joining the IVC just before entry into the right atrium. |
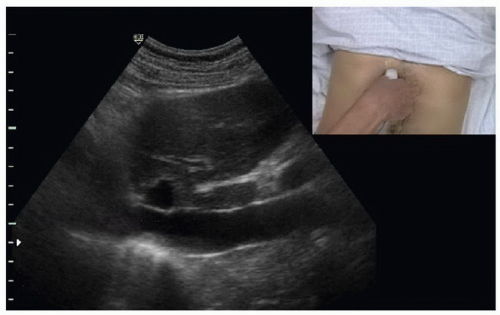
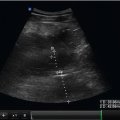
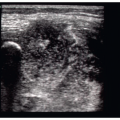
In instances of extreme volume depletion the hepatic segment of the IVC may be completely collapsed and not be easily visualized in a longitudinal axis (Fig. 6.5). In instances where the hepatic segment of the IVC cannot be identified in the longitudinal axis the sonographer can switch to a subxyphoid view of the heart to identify the right atrium (Fig. 6.6). After identifying the right atrium the probe is slid to the patient’s right, bringing the right atrium center on the ultrasound screen. The probe is then angled nearly perpendicular to the skin surface to identify the IVC (Fig. 6.7), followed by a 90-degree clockwise rotation of the probe to turn the probe indicator to the patient’s head and obtain a long-axis view of the IVC (Fig. 6.8). It is worth mentioning however, that if the hepatic segment is not visible secondary to collapse, then clinical concern for low volume status is answered. If clinically indicated, the patient should be bolused with IV fluids followed by repeat ultrasound of the IVC with the patient remaining in the supine position to evaluate for change in diameter.
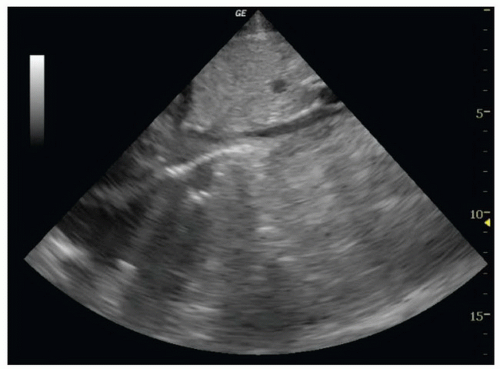 FIGURE 6.4. Long-Axis Image of the IVC Entering the Right Atrium (RA). The hepatic vein inlet is not visualized. |
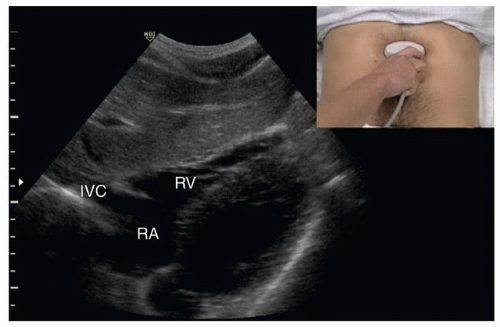 FIGURE 6.6. Subcostal View of the Heart. The right atrium (RA), right ventricle (RV), and inferior vena cava (IVC) are identified. |
Various locations for sampling the IVC diameter have been reported, ranging from just cephalad to the hepatic vein inlet to the inlet of the left renal vein (21,23,31,35, 36, 37, 38, 39, 40, 41, 42, 43, 44). Locations between the hepatic vein inlet and the right atrium are not ideal because the muscular attachment of the diaphragm may result in decreased compliance (44). The most frequent location for sampling is 1 cm distal to the hepatic vein inlet or 2 to 3 cm from the right atrium if the hepatic vein inlet is not visualized. However, Lichtenstein reports a saber profile or bulge of the IVC, caused by the incoming hepatic vein giving concern for unreliable diameter measurements (36). In addition, the collapsibility of the hepatic segment may be influenced by the surrounding parenchyma, with liver fibrosis or cirrhosis causing impaired diminution (35). In instances where the hepatic segment appears to be an unreliable location for diameter sampling, a feasible alternative is the inlet of the left renal vein to the IVC (Fig. 6.9) (45).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree